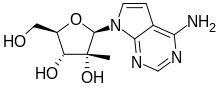
MK-608
 | |
| Clinical data | |
|---|---|
| Other names | 7-Deaza-2’-C-methyladenosine; 7DMA |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H16N4O4 |
| Molar mass | 280.284 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
MK-608 (7-deaza-2’-C-methyladenosine, 7DMA) is an antiviral drug, an adenosine analog (a type of nucleoside analog). It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies,[1][2] it was ultimately unsuccessful in clinical trials.[3] Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever,[4] tick-borne encephalitis virus,[5] poliovirus,[6][7] and most recently Zika virus,[8][9] in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.[10]
See also
References
- ↑ Carroll SS, Ludmerer S, Handt L, Koeplinger K, Zhang NR, Graham D, et al. (March 2009). "Robust antiviral efficacy upon administration of a nucleoside analog to hepatitis C virus-infected chimpanzees". Antimicrobial Agents and Chemotherapy. 53 (3): 926–34. doi:10.1128/AAC.01032-08. PMC 2650549. PMID 19075052.
- ↑ Olsen DB, Davies ME, Handt L, Koeplinger K, Zhang NR, Ludmerer SW, et al. (February 2011). "Sustained viral response in a hepatitis C virus-infected chimpanzee via a combination of direct-acting antiviral agents". Antimicrobial Agents and Chemotherapy. 55 (2): 937–9. doi:10.1128/AAC.00990-10. PMC 3028818. PMID 21115793.
- ↑ Arnold JJ, Sharma SD, Feng JY, Ray AS, Smidansky ED, Kireeva ML, et al. (2012). "Sensitivity of mitochondrial transcription and resistance of RNA polymerase II dependent nuclear transcription to antiviral ribonucleosides". PLOS Pathogens. 8 (11): e1003030. doi:10.1371/journal.ppat.1003030. PMC 3499576. PMID 23166498.
- ↑ Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG (March 2007). "A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs". The Journal of Infectious Diseases. 195 (5): 665–74. doi:10.1086/511310. PMID 17262707.
- ↑ Eyer L, Valdés JJ, Gil VA, Nencka R, Hřebabecký H, Šála M, et al. (September 2015). "Nucleoside inhibitors of tick-borne encephalitis virus". Antimicrobial Agents and Chemotherapy. 59 (9): 5483–93. doi:10.1128/AAC.00807-15. PMC 4538560. PMID 26124166.
- ↑ Goris N, De Palma A, Toussaint JF, Musch I, Neyts J, De Clercq K (March 2007). "2'-C-methylcytidine as a potent and selective inhibitor of the replication of foot-and-mouth disease virus". Antiviral Research. 73 (3): 161–8. doi:10.1016/j.antiviral.2006.09.007. PMID 17055073.
- ↑ Wu R, Smidansky ED, Oh HS, Takhampunya R, Padmanabhan R, Cameron CE, Peterson BR (November 2010). "Synthesis of a 6-methyl-7-deaza analogue of adenosine that potently inhibits replication of polio and dengue viruses". Journal of Medicinal Chemistry. 53 (22): 7958–66. doi:10.1021/jm100593s. PMC 2990348. PMID 20964406.
- ↑ Eyer L, Nencka R, Huvarová I, Palus M, Joao Alves M, Gould EA, et al. (September 2016). "Nucleoside Inhibitors of Zika Virus". The Journal of Infectious Diseases. 214 (5): 707–11. doi:10.1093/infdis/jiw226. PMID 27234417.
- ↑ Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J (May 2016). "The Viral Polymerase Inhibitor 7-Deaza-2'-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model". PLOS Neglected Tropical Diseases. 10 (5): e0004695. doi:10.1371/journal.pntd.0004695. PMC 4862633. PMID 27163257.
- ↑ Vittori S, Dal Ben D, Lambertucci C, Marucci G, Volpini R, Cristalli G (2006). "Antiviral properties of deazaadenine nucleoside derivatives". Current Medicinal Chemistry. 13 (29): 3529–52. doi:10.2174/092986706779026228. PMID 17168721.