Umifenovir
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arbidol |
| Other names | AR-1I9514 |
| Pregnancy category |
|
| Routes of administration | By mouth (hard capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Hepatic |
| Elimination half-life | 17–21 hours |
| Excretion | 40% excrete as unchanged umifenovir in feces (38.9%) and urine (0.12%)[3] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.800 |
| Chemical and physical data | |
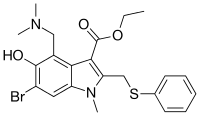
| Formula | C22H25BrN2O3S |
| Molar mass | 477.42 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Umifenovir, sold under the brand name Arbidol, is an antiviral medication for the treatment of influenza infection used in Russia[4] and China. The drug is manufactured by Pharmstandard (Russian: Фармстандарт). Russian and China studies have shown it to be effective and it is approved in both countries while, it is not approved by the US FDA for the treatment or prevention of influenza because it was never applied for FDA approval since the drug company is in Russia not the US.[5]
Chemically, umifenovir features an indole core, functionalized at all but one positions with different substituents. The drug has been shown in studies to inhibit viral entry into target cells [6] and stimulate the immune response.
Umifenovir is manufactured and made available as tablets, capsules and syrup.
Status

Testing of umifenovir's efficacy has mainly occurred in China and Russia,[7][8] and it is well known in these two countries.[9] Some of the Russian tests showed the drug to be effective[7] and a direct comparison with oseltamivir showed similar efficiency in vitro and in a clinical setting.[10] In 2010 Arbidol was the drug brand with the highest sales volume in Russia.[11] In the first quarter of 2020 it had a 16 percent share in the Russian antiviral market.[12]
Mode of action
Biochemistry
Umifenovir inhibits membrane fusion of influenza virus.[4] Umifenovir prevents contact between the virus and target host cells. Fusion between the viral envelope (surrounding the viral capsid) and the cell membrane of the target cell is inhibited. This prevents viral entry to the target cell, and therefore protects it from infection.[13]
Some evidence suggests that the drug's actions are more effective at preventing infections from RNA viruses than infections from DNA viruses.[14]
As well as specific antiviral action against both influenza A and influenza B viruses, umifenovir exhibits modulatory effects on the immune system. The drug stimulates a humoral immune response, induces interferon-production, and stimulates the phagocytic function of macrophages.[15]
Clinical application
Umifenovir is used primarily as an antiviral treatment for influenza. The drug has also been investigated as a candidate drug for treatment of hepatitis C.[16]
More recent studies indicate that umifenovir also has in vitro effectiveness at preventing entry of Zaire ebolavirus (Kikwit strain), Tacaribe arenavirus and Kaposi's sarcoma-associated herpesvirus in mammalian cell cultures, while confirming umifenovir's suppressive effect in vitro on Hepatitis B and poliovirus infection of mammalian cells when introduced either in advance of viral infection or during infection.[17][18]
Research
As of 2020, umifenovir and darunavir were being studied as a potential combination treatment for COVID-19 in China.[19][20][21][22]
Side effects
Side effects in children include sensitization to the drug. No known overdose cases have been reported and allergic reactions are limited to people with hypersensitivity. The LD50 is more than 4 g/kg.[23]
Criticism
In 2007, the Russian Academy of Medical Sciences stated that the effects of Arbidol (umifenovir) are not scientifically proven.[24]
Russian media criticized lobbying attempts by Tatyana Golikova (then-Minister of Healthcare) to promote umifenovir,[25] and the unproven claim that Arbidol can speed up recovery from flu or cold by 1.3-2.3 days.[26] They also made claims that the efficacy of umifenovir is supported by peer-reviewed studies.[27][28]
Names
Arbidol is the INN.[29]
The brand name Arbidol is known as Russian: Арбидол and Chinese: 阿比朵尔.
References
- 1 2 "阿比朵尔抑制新冠,先声药业生产的吗?". 全民健康网 (in Chinese). Archived from the original on 17 June 2020. Retrieved 17 June 2020.
- ↑ "ИНСТРУКЦИЯ ПО ПРИМЕНЕНИЮ АРПЕТОЛ (ARPETOL)". Vidal (in Belarusian). Archived from the original on 26 January 2018. Retrieved 17 June 2020.
- ↑ "Full Prescribing Information: Arbidol (umifenovir) film-coated tablets 50 and 100 mg: Corrections and Additions". State Register of Medicines (in Russian). Open joint-stock company “Pharmstandard-Tomskchempharm”. Retrieved 3 June 2015.
- 1 2 Leneva IA, Russell RJ, Boriskin YS, Hay AJ (February 2009). "Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol". Antiviral Research. 81 (2): 132–40. doi:10.1016/j.antiviral.2008.10.009. PMID 19028526.
- ↑ "FDA Approved Drugs for Influenza". U.S. Food and Drug Administration. 23 October 2020.
- ↑ Kadam, Rameshwar U.; Wilson, Ian A. (2017). "Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol". Proceedings of the National Academy of Sciences. 114 (2): 206–214. doi:10.1073/pnas.1617020114. PMC 5240704. PMID 28003465.
- 1 2 Leneva IA, Fediakina IT, Gus'kova TA, Glushkov RG (2005). "[Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A]". Terapevticheskii Arkhiv (Russian translation). ИЗДАТЕЛЬСТВО "МЕДИЦИНА". 77 (8): 84–8. PMID 16206613.
- ↑ Wang MZ, Cai BQ, Li LY, Lin JT, Su N, Yu HX, Gao H, Zhao JZ, Liu L (June 2004). "[Efficacy and safety of arbidol in treatment of naturally acquired influenza]". Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae. 26 (3): 289–93. PMID 15266832.
- ↑ Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ (2008). "Arbidol: a broad-spectrum antiviral compound that blocks viral fusion". Current Medicinal Chemistry. 15 (10): 997–1005. doi:10.2174/092986708784049658. PMID 18393857.
- ↑ Leneva IA, Burtseva EI, Yatsyshina SB, Fedyakina IT, Kirillova ES, Selkova EP, Osipova E, Maleev VV (February 2016). "Virus susceptibility and clinical effectiveness of anti-influenza drugs during the 2010-2011 influenza season in Russia". International Journal of Infectious Diseases. 43: 77–84. doi:10.1016/j.ijid.2016.01.001. PMID 26775570.
- ↑ "Russian Pharmaceutical Market 2010" (PDF). DSM Group. Archived from the original (PDF) on 20 December 2018. Retrieved 17 June 2020.
- ↑ "Most popular antiviral medication brands in the Russian pharmaceutical market in 1st quarter 2020 by sales value share". Statista. Retrieved 17 June 2020.
- ↑ Boriskin YS, Pécheur EI, Polyak SJ (July 2006). "Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection". Virology Journal. 3: 56. doi:10.1186/1743-422X-3-56. PMC 1559594. PMID 16854226.
- ↑ Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, Hou W, Cheng L, Xiao H, Yang Z (2007). "Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo". Archives of Virology. 152 (8): 1447–55. doi:10.1007/s00705-007-0974-5. PMID 17497238. S2CID 13595688.
- ↑ Glushkov RG, Gus'kova TA, Krylova LIu, Nikolaeva IS (1999). "[Mechanisms of arbidole's immunomodulating action]". Vestnik Rossiiskoi Akademii Meditsinskikh Nauk (in Russian) (3): 36–40. PMID 10222830.
- ↑ Pécheur EI, Lavillette D, Alcaras F, Molle J, Boriskin YS, Roberts M, Cosset FL, Polyak SJ (May 2007). "Biochemical mechanism of hepatitis C virus inhibition by the broad-spectrum antiviral arbidol". Biochemistry. 46 (20): 6050–9. doi:10.1021/bi700181j. PMC 2532706. PMID 17455911.
- ↑ Pécheur EI, Borisevich V, Halfmann P, Morrey JD, Smee DF, Prichard M, Mire CE, Kawaoka Y, Geisbert TW, Polyak SJ (January 2016). "The Synthetic Antiviral Drug Arbidol Inhibits Globally Prevalent Pathogenic Viruses". Journal of Virology. 90 (6): 3086–92. doi:10.1128/JVI.02077-15. PMC 4810626. PMID 26739045.
- ↑ Hulseberg CE, Fénéant L, Szymańska-de Wijs KM, Kessler NP, Nelson EA, Shoemaker CJ, Schmaljohn CS, Polyak SJ, White JM (April 2019). "Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses". Journal of Virology. 93 (8). doi:10.1128/JVI.02185-18. PMC 6450122. PMID 30700611.
- ↑ Ng E (4 February 2020). "Coronavirus: are cocktail therapies for flu and HIV the magic cure?". South China Morning Post.
Bangkok and Hangzhou hospitals put combination remedies to the test.
- ↑ Zheng W, Lau M (4 February 2020). "China's health officials say priority is to stop mild coronavirus cases from getting worse". South China Morning Post.
- ↑ Lu H (January 2020). "Drug treatment options for the 2019-new coronavirus (2019-nCoV)". Bioscience Trends. 14 (1): 69–71. doi:10.5582/bst.2020.01020. PMID 31996494.
- ↑ Jun, Chen; Yun, Ling; Xiuhong, Xi; Ping, Liu; Feng, Li; Tao, Li; Zhiyin, Shang; Mei, Wang; Yinzhong, Shen; Hongzhou, Lu (2020-02-21). "Efficacies of lopinavir/ritonavir and arbidol in the treatment of novel coronavirus pneumonia". Chinese Journal of Infectious Diseases (in Chinese). 38: E008. doi:10.3760/cma.j.cn311365-20200210-00050. ISSN 1000-6680.
- ↑ "АРБИДОЛ (ARBIDOL)". Vidal. Archived from the original on 4 February 2009.
- ↑ "Resolution". Meetings of the Presidium of the Formulary Committee. Russian Academy of Medical Sciences. 16 March 2007.
- ↑ "How do we plant federal ministers". MKRU (in Russian). 21 April 2011.
- ↑ Golunov I (23 December 2013). "13 most popular flu cures: do they work?". Professional Journalism Platform (in Russian).
- ↑ Reuters S. "Stick in the wheel". Esquire (in Russian).
- ↑ "Repetition - the mother of learning". Esquire (in Russian).
- ↑ Recommended INN: List 65., WHO Drug Information, Vol. 25, No. 1, 2011, page 91
External links
- "Umifenovir". Drug Information Portal. U.S. National Library of Medicine.
- "Compendium". Drug Information Portal. Drug Information Portal.