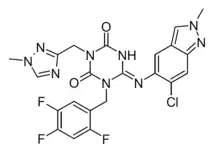
Ensitrelvir
 | |
| Clinical data | |
|---|---|
| Other names | S-217622 |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C22H17ClF3N9O2 |
| Molar mass | 531.88 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ensitrelvir[1] (code name S-217622, brand name Xocova)[2] is an antiviral drug developed by Shionogi in partnership with Hokkaido University, which acts as an orally active 3C-like protease inhibitor for the treatment of COVID-19 infection.[3][4] It is taken by mouth, and has been successfully tested against the recently emerged Omicron variant.[5]
History
It has reached Phase III clinical trials.[3] The Japanese government is reportedly considering allowing Shionogi permission to apply for approval for medical use before the final steps of trials are completed, potentially speeding up the release for sale. This conditional early approval system has previously been used in Japan to accelerate the progression to market of other antiviral drugs targeting COVID-19, including remdesivir and molnupiravir.[6] In a study of 428 patients, viral load was reduced, but symptoms were not significantly reduced. [7]
It became the first Japanese domestic pill to treat COVID-19, third to be regulatorally approved in Japan; in February 2022.[8]
See also
- EDP-235
- Nirmatrelvir
- Tollovir
References
- ↑ World Health Organization (2021). "International Nonproprietary Names for Pharmaceutical Substances. Proposed INN: List 126" (PDF). WHO Drug Information. 35 (4): 1135.
- ↑ Xocova: Powerful New Japanese Pill for Coronavirus Treatment. BioPharma Media, February 2022
- 1 2 Unoh Y, Uehara S, Nakahara K, Nobori H, Yamatsu Y, Yamamoto S, et al. (January 2022). "Discovery of S-217622, a Non-Covalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19". bioRxiv. doi:10.1101/2022.01.26.477782. S2CID 246367525.
- ↑ "Shionogi presents positive Ph II/III results for COVID-19 antiviral S-217622". thepharmaletter.com. 31 January 2022.
- ↑ Shionogi's new COVID pill appears to ease omicron symptoms. Nikkei Asia, 21 December 2021
- ↑ Japan to consider early approval for Shionogi COVID-19 pill. Japan Times, 8 February 2022
- ↑ "Japan's Shionogi seeks approval for COVID-19 pill". Reuters. 25 February 2022.
- ↑ "Japan's Shionogi seeks approval for COVID-19 pill". Reuters. Reuters. 25 February 2022.
| Hepatitis C |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hepatitis D | |||||||||
| Picornavirus |
| ||||||||
| Anti-influenza agents |
| ||||||||
| Multiple/general |
| ||||||||
| |||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||