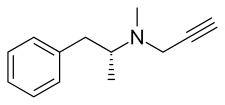
Selegiline
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /səˈlɛdʒɪliːn/ sə-LEJ-i-leen |
| Trade names | Eldepryl, Jumex, Zelapar, Emsam, others[1] |
| Other names | L-Deprenyl; (R)-(–)-N,α-Dimethyl-N-2-propynylphenethylamine; (R)-(–)-N-Methyl-N-2-propynylamphetamine; (R)-(–)-N-2-propynylmethamphetamine |
IUPAC name
| |
| Clinical data | |
| Drug class | Monoamine oxidase inhibitor[2] |
| Main uses | By mouth: Parkinson's[3][2] Patch: Depression[4] |
| Side effects | Nausea, lightheadedness, dry mouth, headache, hallucinations, serotonin syndrome, low blood pressure, urge to gamble, neuroleptic malignant syndrome[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, skin (patch) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697046 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 10% (by mouth), 73% (patch) |
| Protein binding | 94% |
| Metabolism | Intestines and liver |
| Metabolites | N-Desmethylselegiline, levoamphetamine, levomethamphetamine |
| Elimination half-life | 1.5–3.5 hours (oral),[5] 18–25 hours (transdermal)[6] |
| Excretion | Urine |
| Chemical and physical data | |
| Formula | C13H17N |
| Molar mass | 187.281 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Selegiline, sold under the brand names Eldepryl among others, is a medication used to treat parkinsonism including in Parkinson's disease and major depressive disorder.[3][2][4] It is used by mouth or as a patch applied to skin.[2][4]
When taken by mouth, common side effects include nausea, lightheadedness, dry mouth, headache, and hallucinations.[3] When applied as a patch, common side effects include trouble sleeping, rash, and a stuffy nose.[4] Other side effects may include serotonin syndrome, low blood pressure, urge to gamble, neuroleptic malignant syndrome, and sudden onset of sleep.[3] It is a monoamine oxidase B inhibitor at lower doses, while at higher doses it is also a monoamine oxidase A inhibitor.[2][3] It works by increasing brain levels of dopamine and serotonin.[4][3]
Selegilinewas approved for medical use in the United States in 1989.[3] In the United Kingdom 100 tablets of 10 mg costs the NHS about £32 as of 2021.[2] This amount in the United States costs about 130 USD.[7] In the United States a month of patches costs about 1,800 USD.[8]
Medical uses
Parkinson's disease
In its pill form, selegiline is used to treat symptoms of Parkinson's disease.[9] It is most often used as an adjunct to drugs such as levodopa (L-DOPA), although it has been used off-label as a monotherapy.[10][11] The rationale for adding selegiline to levodopa is to decrease the required dose of levodopa and thus reduce the motor complications of levodopa therapy.[12] Selegiline delays the point when levodopa treatment becomes necessary from about 11 months to about 18 months after diagnosis.[13] There is some evidence that selegiline acts as a neuroprotectant and reduces the rate of disease progression, though this is disputed.[11][12]
Depression
Selegiline is also delivered via a transdermal patch used as a treatment for major depressive disorder.[14][15] Administration of transdermal selegiline bypasses hepatic first pass metabolism. This avoids an inhibition of gastrointestinal and hepatic MAO-A activity resulting in an increase in blood of food-borne tyramine and possible adverse effects, while sufficient amount of selegiline reach the brain for an antidepressant effect.[16]
A quantitative review published in 2015 found that for the pooled results of the pivotal trials, the number needed to treat (a sign of effect size, so a low number is better) for the patch for symptom reduction was 11, and for remission, was 9.[15] The number needed to harm (inverse of the NNT, a high number here is better) ranged from 387 for sexual side effects to 7 for application site reaction.[15] With regard to the likelihood to be helped or harmed (LHH), the analysis showed that the selegiline patch was 3.6 times as likely to lead to a remission vs. a discontinuation due to side effects; the LHH for remission vs. incidence of insomnia was 2.1; the LHH for remission vs. discontinuation due to insomnia was 32.7. The LHH for remission vs. insomnia and sexual dysfunction were both very low.[15]
Other
Selegiline has been used in the treatment of attention deficit hyperactivity disorder (ADHD).[17] Selegiline may target specific symptoms of ADHD including: sustained attention, the learning of novel information, hyperactivity, and peer interactions. Selegiline has shown to be relatively effective in treating the inattention subtype of ADHD.[18]
It has been used as palliative treatment for dementia in Alzheimer's disease with unclear benefit.[11]
Side effects
Side effects of the tablet form in conjunction with levodopa include, in decreasing order of frequency, nausea, hallucinations, confusion, depression, loss of balance, insomnia, increased involuntary movements, agitation, slow or irregular heart rate, delusions, hypertension, new or increased angina pectoris, and syncope.[9] Most of the side effects are due to a high dopamine signaling, and can be alleviated by reducing the dose of levodopa.[1]
The main side effects of the patch form for depression include application-site reactions, insomnia, diarrhea, and sore throat.[14] The selegiline patch carries a black box warning about a possible increased risk of suicide, especially for young people,[14] as do all antidepressants since 2007.[19]
Pregnancy
For all human uses and all forms, selegiline is pregnancy category C: studies in pregnant lab animals have shown side effects on the fetus but there are no adequate studies in humans.[9][14]
Interactions
Both the oral and patch forms come with strong warnings against combining selegiline with drugs that could produce serotonin syndrome, such as SSRIs and the cough medicine dextromethorphan.[9][14][20] Selegiline in combination with the opioid analgesic pethidine is not recommended, as it can lead to severe adverse effects.[20] Several other synthetic opioids such as tramadol and methadone, as well as various triptans, are contraindicated due to potential for serotonin syndrome.[21][22]
Birth control pills containing ethinylestradiol and a progestin increase the bioavailability of selegiline by 10- to 20-fold.[23] High levels can lead to loss of MAO-B selectivity, and selegiline may begin inhibition MAO-A as well. This increases susceptibility to side effects of non-selective MAOIs, such as tyramine-induced hypertensive crisis and serotonin toxicity when combined with serotonergic medications.[23]
Both forms of the drug carry warnings about food restrictions to avoid hypertensive crisis that are associated with MAO inhibitors.[9][14] The patch form was created in part to overcome food restrictions; clinical trials showed that it was successful. Additionally, in post-marketing surveillance from April 2006 to October 2010, only 13 self-reports of possible hypertensive events or hypertension were made out of 29,141 exposures to the drug, and none were accompanied by objective clinical data.[15] The lowest dose of the patch method of delivery, 6 mg/24 hours, does not require any dietary restrictions.[24] Higher doses of the patch and oral formulations, whether in combination with the older non-selective MAOIs or in combination with the reversible MAO-A inhibitor moclobemide, require a low-tyramine diet.[20]
Pharmacology
Pharmacodynamics
Selegiline is a selective inhibitor of MAO-B, irreversibly inhibiting it by binding to it covalently.[1][25] It exerts effects by blocking the breakdown of dopamine, thus increasing its activity.[26] Its possible neuroprotective properties may be due to protecting nearby neurons from the free oxygen radicals that are released by MAO-B activity. At higher doses, selegiline loses its selectivity for MAO-B and inhibits MAO-A as well.[1]
Selegiline also inhibits CYP2A6 and can increase the effects of nicotine as a result.[27] Selegiline also appears to activate σ1 receptors, having a relatively high affinity for these receptors of approximately 400 nM.[28][29]
Pharmacokinetics
Selegiline has an oral bioavailability of about 10%, which increases when ingested together with a fatty meal, as the molecule is fat soluble.[1][30] Selegiline and its metabolites bind extensively to plasma proteins (at a rate of 94%). They cross the blood–brain barrier and enter the brain, where they most concentrated at the thalamus, basal ganglia, midbrain, and cingulate gyrus.[11][14]
Selegiline is mostly metabolized in the intestines and liver; it and its metabolites are excreted in the urine.[1]
Buccal administration of selegiline results in 5-fold higher bioavailability, more reproducible blood concentration, and produces less amphetamine metabolites than the oral tablet form.[31]
Metabolism
Selegiline is metabolized by cytochrome P450 to L-desmethylselegiline and levomethamphetamine.[32][33] Desmethylselegiline has some activity against MAO-B, but much less than that of selegiline.[26][25] It is thought to be further metabolized by CYP2C19.[34] Levomethamphetamine is converted to levoamphetamine.
Due to the presence of these metabolites, people taking selegiline may test positive for "amphetamine" or "methamphetamine" on drug screening tests.[35] While the amphetamine metabolites may contribute to selegiline's ability to inhibit reuptake of the neurotransmitters dopamine and norepinephrine, they have also been associated with orthostatic hypotension and hallucinations.[33][36][37] The amphetamine metabolites are hydroxylated and, in phase II, conjugated by glucuronyltransferase.
A newer anti-Parkinson MAO-B inhibitor, rasagiline, metabolizes into 1(R)-aminoindan, which has no amphetamine-like characteristics.[38]
Patch
Following application of the patch to humans, an average of 25% to 30% of the selegiline content is delivered systemically over 24 hours. Transdermal dosing results in significantly higher exposure to selegiline and lower exposure to all metabolites when compared to oral dosing; this is due to the extensive first-pass metabolism of the pill form and low first-pass metabolism of the patch form. The site of application is not a significant factor in how the drug is distributed. In humans, selegiline does not accumulate in the skin, nor is it metabolized there.[14]
Chemistry
Selegiline belongs to the phenethylamine and amphetamine chemical families. It is also known as L-deprenyl, as well as (R)-(–)-N,α-dimethyl-N-(2-propynyl)phenethylamine or (R)-(–)-N-methyl-N-2-propynylamphetamine. The compound is a derivative of levomethamphetamine (L-methamphetamine) with a propargyl group attached to the nitrogen atom. This detail is borrowed from pargyline, an older MAO-B inhibitor of the phenethylamine group.[39] Selegiline is the levorotatory enantiomer of the racemic mixture deprenyl.
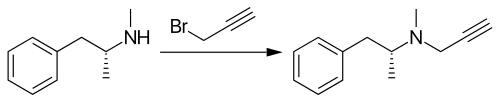
Selegiline is synthesized by the alkylation of (–)-methamphetamine using propargyl bromide.[40][41][42][43]
Another clinically used MAOI of the amphetamine class is tranylcypromine.
History
Following the discovery that the tuberculosis drug iproniazid elevated the mood of people taking it, and the subsequent discovery that the effect was likely due to inhibition of MAO, many people and companies started trying to discover MAO inhibitors to use as antidepressants. Selegiline was discovered by Zoltan Ecseri at the Hungarian drug company, Chinoin (part of Sanofi since 1993),[44] which they called E-250.[45]: 66–67 Chinoin received a patent on the drug in 1962 and the compound was first published in the scientific literature in English in 1965.[45]: 67 [46] Work on the biology and effects of E-250 in animals and humans was conducted by a group led by József Knoll at Semmelweis University which was also in Budapest.[45]: 67

Deprenyl is a racemic compound a mixture of two isomers called enantiomers. Further work determined that the levorotatory enantiomer was a more potent MAO-inhibitor, which was published in 1967, and subsequent work was done with the single enantiomer L-deprenyl.[45]: 67 [47][48]
In 1971, Knoll showed that selegiline selectively inhibits the B-isoform of monoamine oxidase (MAO-B) and proposed that it is unlikely to cause the infamous "cheese effect" (hypertensive crisis resulting from consuming foods containing tyramine) that occurs with non-selective MAO inhibitors. A few years later, two Parkinson's disease researchers based in Vienna, Peter Riederer and Walther Birkmayer, realized that selegiline could be useful in Parkinson's disease. One of their colleagues, Prof. Moussa B.H. Youdim, visited Knoll in Budapest and took selegiline from him to Vienna. In 1975, Birkmayer's group published the first paper on the effect of selegiline in Parkinson's disease.[48][49]
In the 1970s there was speculation that it could be useful as an anti-aging drug or aphrodisiac.[50]
In 1987 Somerset Pharmaceuticals in New Jersey, which had acquired the US rights to develop selegiline, filed a new drug application (NDA) with the FDA to market the drug for Parkinson's disease in the US.[51] While the NDA was under review, Somerset was acquired in a joint venture by two generic drug companies, Mylan and Bolan Pharmaceuticals.[51] Selegiline was approved for Parkinson's disease by the FDA in 1989.[51]
In the 1990s, J. Alexander Bodkin at McLean Hospital, an affiliate of Harvard Medical School, began a collaboration with Somerset to develop delivery of selegiline via a transdermal patch in order to avoid the well known dietary restrictions of MAO inhibitors.[50][52][53] Somerset obtained FDA approval to market the patch in 2006.[54]
Society and culture
In E for Ecstasy (a book examining the uses of the street drug ecstasy in the UK) the writer, activist and ecstasy advocate Nicholas Saunders highlighted test results showing that certain consignments of the drug also contained selegiline.[55] Consignments of ecstasy known as "Strawberry" contained what Saunders described as a "potentially dangerous combination of ketamine, ephedrine and selegiline," as did a consignment of "Sitting Duck" Ecstasy tablets.[56]
David Pearce wrote The Hedonistic Imperative[57] six weeks later after starting taking selegiline.[58]
In Gregg Hurwitz's novel Out of the Dark,[59] selegiline (Emsam) and tyramine-containing food were used to assassinate the president of the United States.
Veterinary use
In veterinary medicine, selegiline is sold under the brand name Anipryl (manufactured by Zoetis). It is used in dogs to treat canine cognitive dysfunction and, at higher doses, pituitary-dependent hyperadrenocorticism (PDH).[60][61] Canine cognitive dysfunction is a form of dementia that mimics Alzheimer's disease in humans. Geriatric dogs treated with selegiline show improvements in sleeping pattern, reduced incontinence, and increased activity level; most show improvements by one month.[62][63] Though it is labeled for dog use only, selegiline has been used off-label for geriatric cats with cognitive dysfunction.[64]
Selegiline's efficacy in treating pituitary-dependent hyperadrenocorticism has been disputed.[60] Theoretically, it works by increasing dopamine levels, which downregulates the release of ACTH, eventually leading to reduced levels of cortisol.[64] Some claim that selegiline is only effective at treating PDH caused by lesions in the anterior pituitary (which comprise most canine cases).[65] The greatest sign of improvement is lessening of abdominal distention.[62]
Side effects in dogs are uncommon, but they include vomiting, diarrhea, diminished hearing, salivation, decreased weight and behavioral changes such as hyperactivity, listlessness, disorientation, and repetitive motions.[61][65]
Selegiline does not appear to have a clinical effect on horses.[65]
References
- 1 2 3 4 5 6 "Selegiline". Drugs.com. Archived from the original on December 29, 2019. Retrieved February 7, 2016.
- 1 2 3 4 5 6 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 447. ISBN 978-0-85711-369-6.
- 1 2 3 4 5 6 7 8 "Selegiline Monograph for Professionals". Drugs.com. Archived from the original on August 13, 2020. Retrieved October 11, 2021.
- 1 2 3 4 5 "EMSAM® (selegiline transdermal system)" (PDF). Archived (PDF) from the original on August 10, 2021. Retrieved October 11, 2021.
- ↑ "Archive copy". Archived from the original on April 16, 2015. Retrieved July 10, 2021.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archive copy" (PDF). Archived (PDF) from the original on April 3, 2021. Retrieved July 10, 2021.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Selegiline Prices and Selegiline Coupons - GoodRx". GoodRx. Retrieved October 11, 2021.
- ↑ "Emsam Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on November 15, 2016. Retrieved October 11, 2021.
- 1 2 3 4 5 Selegiline oral label Archived June 21, 2020, at the Wayback Machine. Updated December 31, 2008
- ↑ Riederer P, Lachenmayer L, Laux G (August 2004). "Clinical applications of MAO-inhibitors". Current Medicinal Chemistry. 11 (15): 2033–43. doi:10.2174/0929867043364775. PMID 15279566.
- 1 2 3 4 "Selegiline Hydrochloride Monograph for Professionals". Drugs.com. Archived from the original on August 13, 2020. Retrieved February 23, 2018.
- 1 2 Ives NJ, Stowe RL, Marro J, Counsell C, Macleod A, Clarke CE, et al. (September 2004). "Monoamine oxidase type B inhibitors in early Parkinson's disease: meta-analysis of 17 randomised trials involving 3525 patients". BMJ. 329 (7466): 593. doi:10.1136/bmj.38184.606169.AE. PMC 516655. PMID 15310558.
- ↑ Riederer P, Lachenmayer L (November 2003). "Selegiline's neuroprotective capacity revisited". Journal of Neural Transmission. 110 (11): 1273–8. doi:10.1007/s00702-003-0083-x. PMID 14628191. S2CID 20232921.
- 1 2 3 4 5 6 7 8 Emsam label Archived April 1, 2021, at the Wayback Machine Last revised Sept 2014. Index page at FDA Archived August 29, 2021, at the Wayback Machine
- 1 2 3 4 5 Citrome L, Goldberg JF, Portland KB (November 2013). "Placing transdermal selegiline for major depressive disorder into clinical context: number needed to treat, number needed to harm, and likelihood to be helped or harmed". Journal of Affective Disorders. 151 (2): 409–17. doi:10.1016/j.jad.2013.06.027. PMID 23890583.
- ↑ Lee KC, Chen JJ (November 2007). "Transdermal selegiline for the treatment of major depressive disorder". Neuropsychiatr. Dis. Treat. 3 (5): 527–37. PMID 19300583.
- ↑ Moore JJ, Saadabadi A (January 2020). "Selegiline". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 30252350.
- ↑ "Placebo-controlled study examining effects of selegiline in children with attention-deficit/hyperactivity disorder". pubmed.gov. Archived from the original on May 25, 2021. Retrieved July 10, 2021.
- ↑ Friedman RA, Leon AC (June 2007). "Expanding the black box - depression, antidepressants, and the risk of suicide". The New England Journal of Medicine. 356 (23): 2343–6. doi:10.1056/NEJMp078015. PMID 17485726.
- 1 2 3 Heinonen EH, Myllylä V (July 1998). "Safety of selegiline (deprenyl) in the treatment of Parkinson's disease". Drug Safety. 19 (1): 11–22. doi:10.2165/00002018-199819010-00002. PMID 9673855. S2CID 9632549.
- ↑ Csoti I, Storch A, Müller W, Jost WH (December 1, 2012). "Drug interactions with selegiline versus rasagiline". Basal Ganglia. Monoamine oxidase B Inhibitors. 2 (4, Supplement): S27–S31. doi:10.1016/j.baga.2012.06.003. ISSN 2210-5336.
- ↑ Gillman PK (October 2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–41. doi:10.1093/bja/aei210. PMID 16051647.
- 1 2 Laine K, Anttila M, Helminen A, Karnani H, Huupponen R (March 1999). "Dose linearity study of selegiline pharmacokinetics after oral administration: evidence for strong drug interaction with female sex steroids". British Journal of Clinical Pharmacology. 47 (3): 249–54. doi:10.1046/j.1365-2125.1999.00891.x. PMC 2014223. PMID 10215747.
- ↑ Jessen L, Kovalick LJ, Azzaro AJ (April 2008). "The selegiline transdermal system (emsam): a therapeutic option for the treatment of major depressive disorder". P & T. 33 (4): 212–46. PMC 2730099. PMID 19750165.
- 1 2 Factor SA, Weiner W (2007). Parkinson's Disease: Diagnosis & Clinical Management (2nd ed.). Demos Medical Publishing. pp. 503, 505. ISBN 978-1-934559-87-1. Archived from the original on December 10, 2020. Retrieved July 10, 2021.
- 1 2 Katzung BG (2004). Basic and Clinical Pharmacology (9th ed.). Lange Medical Books/McGraw Hill. pp. 453. ISBN 978-0-07-141092-2.
- ↑ Siu EC, Tyndale RF (March 2008). "Selegiline is a mechanism-based inactivator of CYP2A6 inhibiting nicotine metabolism in humans and mice". The Journal of Pharmacology and Experimental Therapeutics. 324 (3): 992–9. doi:10.1124/jpet.107.133900. PMID 18065502.
- ↑ Itzhak Y (1994). Sigma Receptors. Academic Press. p. 84. ISBN 978-0-12-376350-1.
- ↑ Stone TW (1993). Acetylcholine, Sigma Receptors, CCK and Eicosanoids, Neurotoxins. Taylor & Francis. p. 124. ISBN 978-0-7484-0063-8.
- ↑ Barrett JS, Szego P, Rohatagi S, Morales RJ, De Witt KE, Rajewski G, Ireland J (October 1996). "Absorption and presystemic metabolism of selegiline hydrochloride at different regions in the gastrointestinal tract in healthy males". Pharmaceutical Research. 13 (10): 1535–40. doi:10.1023/A:1016035730754. PMID 8899847. S2CID 24654277.
- ↑ Clarke A, Brewer F, Johnson ES, Mallard N, Hartig F, Taylor S, Corn TH (November 2003). "A new formulation of selegiline: improved bioavailability and selectivity for MAO-B inhibition". Journal of Neural Transmission. 110 (11): 1241–55. doi:10.1007/s00702-003-0036-4. PMID 14628189. S2CID 711419.
- ↑ Engberg G, Elebring T, Nissbrandt H (November 1991). "Deprenyl (selegiline), a selective MAO-B inhibitor with active metabolites; effects on locomotor activity, dopaminergic neurotransmission and firing rate of nigral dopamine neurons". The Journal of Pharmacology and Experimental Therapeutics. 259 (2): 841–7. PMID 1658311.
- 1 2 Lemke TL, Williams DA, eds. (2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 434. ISBN 978-1609133450.
- ↑ Taavitsainen P, Anttila M, Nyman L, Karnani H, Salonen JS, Pelkonen O (May 2000). "Selegiline metabolism and cytochrome P450 enzymes: in vitro study in human liver microsomes". Pharmacology & Toxicology. 86 (5): 215–21. doi:10.1034/j.1600-0773.2000.pto860504.x. PMID 10862503.
- ↑ Romberg RW, Needleman SB, Snyder JJ, Greedan A (November 1995). "Methamphetamine and amphetamine derived from the metabolism of selegiline". Journal of Forensic Sciences. 40 (6): 1100–2. doi:10.1520/JFS13885J. PMID 8522918.
- ↑ Bar Am O, Amit T, Youdim MB (January 2004). "Contrasting neuroprotective and neurotoxic actions of respective metabolites of anti-Parkinson drugs rasagiline and selegiline". Neuroscience Letters. 355 (3): 169–72. doi:10.1016/j.neulet.2003.10.067. PMID 14732458. S2CID 20471004.
- ↑ Yasar S, Goldberg JP, Goldberg SR (January 1, 1996). "Are metabolites of l-deprenyl (selegiline) useful or harmful? Indications from preclinical research". Journal of Neural Transmission. Supplementum. 48: 61–73. doi:10.1007/978-3-7091-7494-4_6. ISBN 978-3-211-82891-5. PMID 8988462.
- ↑ Chen JJ, Swope DM (August 2005). "Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of Parkinson disease". Journal of Clinical Pharmacology. 45 (8): 878–94. doi:10.1177/0091270005277935. PMID 16027398. S2CID 24350277. Archived from the original on July 11, 2012.
- ↑ Miklya I (March 13, 2014). "The History of Selegiline/(-)-Deprenyl the First Selective Inhibitor of B-Type Monoamine Oxidase and The First Synthetic Catecholaminergic Activity Enhancer Substance". International Network for the History of Neuropsychopharmacology. Archived from the original on February 7, 2016. Retrieved January 7, 2016.
- ↑ J. Knoll, E. Sanfai, DE 1568277 (1966).
- ↑ J. Hermann Nee Voeroes, Z. Ecsery, G. Sabo, L. Arvai, L. Nagi, O. Orban, E. Sanfai, U.S. Patent 4,564,706 (1986)
- ↑ B. Brunova, M. Ferenc, EP 344675 (1989)
- ↑ Fowler JS (July 1977). "2-Methyl-3-butyn-2-ol as an acetylene precursor in the Mannich reaction. A new synthesis of suicide inactivators of monoamine oxidase". The Journal of Organic Chemistry. 42 (15): 2637–7. doi:10.1021/jo00435a026. PMID 874623.
- ↑ "Sanofi Extends Holding in Chinoin". The Pharma Letter. September 19, 1993. Archived from the original on December 27, 2019. Retrieved July 10, 2021.
- 1 2 3 4 Magyar K (2011). "The pharmacology of selegiline". In Youdim M, Riederer P (eds.). Monoamine Oxidases and Their Inhibitors. International Review of Neurobiology. Vol. 100. Academic Press. ISBN 978-0-12-386468-0. Archived from the original on December 26, 2019. Retrieved July 10, 2021.
- ↑ Knoll J, Ecseri Z, Kelemen K, Nievel J, Knoll B (May 1965). "Phenylisopropylmethylpropinylamine (E-250), a new spectrum psychic energizer". Archives Internationales de Pharmacodynamie et de Therapie. 155 (1): 154–64. PMID 4378644.
- ↑ Magyar K, Vizi ES, Ecseri Z, Knoll J (1967). "Comparative pharmacological analysis of the optical isomers of phenyl-isopropyl-methyl-propinylamine (E-250)". Acta Physiologica Academiae Scientiarum Hungaricae. 32 (4): 377–87. PMID 5595908.
- 1 2 Healy D (2000). "The psychopharmacology of life and death. Interview with Joseph Knoll.". The Psychopharmacologists, Vol. III: Interviews. London: Arnold. pp. 81–110. ISBN 978-0-340-76110-6.
- ↑ Birkmayer W, Riederer P, Youdim MB, Linauer W (1975). "The potentiation of the anti akinetic effect after L-dopa treatment by an inhibitor of MAO-B, Deprenil". Journal of Neural Transmission. 36 (3–4): 303–26. doi:10.1007/BF01253131. PMID 1172524. S2CID 38179089. Archived from the original on February 12, 2013.
- 1 2 Cromie WJ (November 7, 2002). "Bodkin is Patching up Depression". Harvard University Gazette. Archived from the original on February 24, 2018. Retrieved September 8, 2007.
- 1 2 3 Seaman JT, Landry JT (2011). Mylan: 50 Years of Unconventional Success: Making Quality Medicine Affordable and Accessible. University Press of New England. p. 50. ISBN 978-1-61168-269-4.
- ↑ Frampton JE, Plosker GL (2007). "Selegiline transdermal system: in the treatment of major depressive disorder". Drugs. 67 (2): 257–65, discussion 266–7. doi:10.2165/00003495-200767020-00006. PMID 17284087. S2CID 42425086.
- ↑ Duffy M (December 3, 2002). "Patch Raises New Hope For Beating Depression". The New York Times. ISSN 0362-4331. Archived from the original on May 6, 2021. Retrieved July 10, 2021.
- ↑ Cascade EF, Kalali AH, Preskorn SH (June 2007). "Emsam: the first year". Psychiatry. 4 (6): 19–21. PMC 2921248. PMID 20711332.
- ↑ Saunders N, Heron L (1993). E for Ecstasy. London: N. Saunders. ISBN 978-0-9501628-8-1. OCLC 29388575.
- ↑ Saunders N. "Test results of 30 samples of Ecstasy bought in British clubs between 11/94 and 7/95". Archived from the original on April 17, 2020. Retrieved July 10, 2021.
- ↑ Pearce, David (1995). The Hedonistic Imperative. OCLC 44325836. Archived from the original on May 21, 2021. Retrieved July 10, 2021.
- ↑ "Sam Barker and David Pearce on Art, Paradise Engineering, and Existential Hope (With Guest Mix) | The FLI Podcast". Future of Life Institute (audio, transcript). June 24, 2020. Archived from the original on July 6, 2021. Retrieved July 10, 2021.
- ↑ Hurwitz, Gregg (2019). Out of the dark. p. 431. ISBN 9780718185480.
- 1 2 Braddock JA, Church DB, Robertson ID (2004). "Selegiline Treatment of Canine Pituitary-Dependent Hyperadrenocorticism" (PDF). Australian Veterinary Journal. Archived from the original (PDF) on November 29, 2010. Retrieved April 8, 2011. (PDF)
- 1 2 Eghianruwa K (2014). Essential Drug Data for Rational Therapy in Veterinary Practice. AuthorHouse. pp. 127–128. ISBN 978-1-4918-0010-2. Archived from the original on December 27, 2019. Retrieved July 10, 2021.
- 1 2 "Anipryl Tablets for Animal Use". Drugs.com. Archived from the original on September 1, 2017. Retrieved August 31, 2017.
- ↑ Lundgren B. "Canine Cognitive Dysfunction". Veterinary Partner. Archived from the original on March 10, 2011. Retrieved April 8, 2011.
- 1 2 Riviere JE, Papich MG (2013). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 530. ISBN 978-1-118-68590-7. Archived from the original on June 14, 2020. Retrieved July 10, 2021.
- 1 2 3 Papich MG (2015). Saunders Handbook of Veterinary Drugs: Small and Large Animal. Elsevier Health Sciences. p. 722. ISBN 978-0-323-24485-5. Archived from the original on December 25, 2019. Retrieved July 10, 2021.
External links
| Identifiers: |
|
|---|