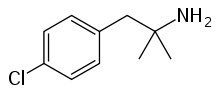
Chlorphentermine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | p-Chloro-α,α-dimethylphenethylamine |
| Routes of administration | Oral, Insufflated, Rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 40 hours |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.651 |
| Chemical and physical data | |
| Formula | C10H14ClN |
| Molar mass | 183.68 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Chlorphentermine (trade names Apsedon, Desopimon, Lucofen) is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the 4-chloro derivative of the better known appetite suppressant phentermine,[1] which is still in current use.
Chlorphentermine acts as a highly selective serotonin releasing agent (SRA).[2] It is not a psychostimulant and has little or no abuse potential, but is classed as a Schedule III drug in the USA due mainly to its similarity to other appetite suppressants such as diethylpropion which have been more widely abused. It is no longer used due mainly to safety concerns, as it has a serotonergic effects profile similar to other withdrawn appetite suppressants such as fenfluramine and aminorex which were found to cause pulmonary hypertension and cardiac fibrosis following prolonged use.[3]
The plasma half-life is about five days.[4] It was withdrawn from the market in the UK in 1974.[4]
See also
References
- ↑ Gylys JA, Hart JJ, Warren MR (September 1962). "Chlorphentermine, a new anorectic agent". The Journal of Pharmacology and Experimental Therapeutics. 137: 365–73. PMID 13903304.
- ↑ Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ↑ Rothman RB, Ayestas MA, Dersch CM, Baumann MH (August 1999). "Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension". Circulation. 100 (8): 869–75. doi:10.1161/01.cir.100.8.869. PMID 10458725.
- 1 2 Craddock D (1976). "Anorectic drugs: use in general practice". Drugs. 11 (5): 378–93. doi:10.2165/00003495-197611050-00002. PMID 782835. S2CID 25704474.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|