Dimethylglycine
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Dimethylamino)acetic acid | |
| Other names
N,N-Dimethylglycine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| 3DMet | |
Beilstein Reference |
1700261 |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.012.971 |
| EC Number |
|
Gmelin Reference |
82215 |
| KEGG | |
| MeSH | dimethylglycine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C4H9NO2 |
| Molar mass | 103.121 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Density | 1.069 g/mL |
| Melting point | 178 to 182 °C (352 to 360 °F; 451 to 455 K) |
| Boiling point | 175.2 °C (347.4 °F; 448.3 K) |
| Hazards | |
| GHS labelling: | |
Pictograms |
 |
Signal word |
Warning |
Hazard statements |
H302 |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
>650 mg kg−1 (oral, rat) |
| Related compounds | |
Related alkanoic acids |
|
Related compounds |
Dimethylacetamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
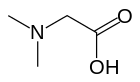
Dimethylglycine (DMG) is a derivative of the amino acid glycine with the structural formula (CH3)2NCH2COOH. It can be found in beans and liver. It can be formed from trimethylglycine upon the loss of one of its methyl groups. It is also a byproduct of the metabolism of choline.
When DMG was first discovered, it was referred to as Vitamin B16, but, unlike true B vitamins, deficiency of DMG in the diet does not lead to any ill-effects and it is synthesized by the human body in the citric acid (or Krebs) cycle meaning it does not meet the definition of a vitamin.
Uses
Dimethylglycine has been suggested for use as an athletic performance enhancer, immunostimulant, and a treatment for autism, epilepsy, or mitochondrial disease.[1] There is no evidence that dimethylglycine is effective for treating mitochondrial disease.[2] Published studies on the subject have shown little to no difference between DMG treatment and placebo in autism spectrum disorders.[3][4]
Biological activity
Dimethylglycine has been found to act as an agonist of the glycine site of the NMDA receptor.[5]
Preparation
This compound is commercially available as the free form amino acid, and as the hydrochloride salt [2491-06-7 ]. DMG may be prepared by the alkylation of glycine via the Eschweiler–Clarke reaction. In this reaction, glycine is treated with aqueous formaldehyde in formic acid that serves as both solvent and reductant. Hydrochloric acid is added thereafter to give the hydrochloride salt. The free amino acid may have been obtained by neutralization of the acid salt, which has been performed with silver oxide.[6]
- H2NCH2COOH + 2 CH2O + 2 HCOOH → (CH3)2NCH2COOH + 2 CO2 + 2 H2O
References
- ↑ "Dimethylglycine". About Herbs, Botanicals & Other Products. Memorial Sloan–Kettering Cancer Center. December 8, 2009.
- ↑ Pfeffer, Gerald; Majamaa, Kari; Turnbull, Douglass M.; Thorburn, David; Chinnery, Patrick F. (2012-04-18). "Treatment for mitochondrial disorders". The Cochrane Database of Systematic Reviews (4): CD004426. doi:10.1002/14651858.CD004426.pub3. ISSN 1469-493X. PMC 7201312. PMID 22513923.
- ↑ Bolman WM, Richmond JA (June 1999). "A double-blind, placebo-controlled, crossover pilot trial of low-dose dimethylglycine in patients with autistic disorder". Journal of Autism and Developmental Disorders. 29 (3): 191–4. doi:10.1023/A:1023023820671. PMID 10425581. S2CID 6993427.
- ↑ Kern JK, Miller VS, Cauller PL, Kendall PR, Mehta PJ, Dodd M (March 2001). "Effectiveness of N,N-dimethylglycine in autism and pervasive developmental disorder". Journal of Child Neurology. 16 (3): 169–73. doi:10.1177/088307380101600303. PMID 11305684. S2CID 25016913.
- ↑ Lin, Jen-Cheng; Chan, Ming-Huan; Lee, Mei-Yi; Chen, Yi-Chyan; Chen, Hwei-Hsien (2016). "N,N-dimethylglycine differentially modulates psychotomimetic and antidepressant-like effects of ketamine in mice". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 71: 7–13. doi:10.1016/j.pnpbp.2016.06.002. ISSN 0278-5846. PMID 27296677. S2CID 4817868.
- ↑ Clarke, H. T.; Gillespie, H. B.; Weisshaus, S. Z. (1933). "The Action of Formaldehyde on Amines and Amino Acids". Journal of the American Chemical Society. 55 (11): 4571. doi:10.1021/ja01338a041.