Levorphanol
 | |
 | |
| Names | |
|---|---|
| Trade names | Levo-Dromoran |
| Other names | Ro 1-5431[1] |
IUPAC name
| |
| Clinical data | |
| Drug class | Opioid |
| Main uses | Moderate to severe pain[2] |
| Side effects | Nausea, flushing, urinary retention, itchiness, constipation, respiratory depression[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Onset of action | With 1 hr[3] |
| Duration of action | Up to 8 hrs[2] |
| Typical dose | 2 mg TID to QID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682020 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 70% (by mouth); 100% (IV) |
| Protein binding | 40% |
| Metabolism | Liver |
| Elimination half-life | 11–16 hours |
| Chemical and physical data | |
| Formula | C17H23NO |
| Molar mass | 257.377 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Levorphanol, sold under the brand name Levo-Dromoran, is an opioid medication used to treat moderate to severe pain.[2] It is taken by mouth.[2] Maximum effects occur within an hour and last up to 8 hours.[3][2]
Common side effects include nausea, flushing, urinary retention, itchiness, and constipation.[2] Other side effects may include insufficient breathing (respiratory depression), abuse, serotonin syndrome, and low blood pressure.[2] Safety in pregnancy is unclear.[4]
Levorphanol was first described in Germany in 1946.[5] It has been in medical use in the United States since 1953.[6] In the United States 90 tablets of 2 mg cost about 1,100 USD as of 2021.[7] In the United States it is a Schedule II controlled substance.[2]
Medical uses
Dosage
It is generally started at 2 mg three to four times per day.[2]
Pharmacology
Levorphanol acts predominantly as an agonist of the μ-opioid receptor (MOR), but is also an agonist of the δ-opioid receptor (DOR), κ-opioid receptor (KOR), and the nociceptin receptor (NOP), as well as an NMDA receptor antagonist and a serotonin-norepinephrine reuptake inhibitor (SNRI).[6] Levorphanol, similarly to certain other opioids, also acts as a glycine receptor antagonist and GABA receptor antagonist at very high concentrations.[8] Levorphanol is 6 to 8 times as potent as morphine at the MOR.
Relative to morphine, levorphanol lacks complete cross-tolerance[9] and possesses greater intrinsic activity at the MOR.[9] The duration of action is generally long compared to other comparable analgesics and varies from 4 hours to as much as 15 hours. For this reason levorphanol is useful in palliation of chronic pain and similar conditions. Levorphanol has an oral to parenteral effectiveness ratio of 2:1, one of the most favorable of the strong narcotics. Its antagonism of the NMDA receptor, similar to those of the phenylheptylamine open-chain opioids such as methadone or the phenylpiperidine ketobemidone, make levorphanol useful for types of pain that other analgesics may not be as effective against, such as neuropathic pain.[10] Levorphanol's exceptionally high analgesic efficacy in the treatment of neuropathic pain is also conferred by its action on serotonin and norepinephrine transporters, similar to the opioids tramadol and tapentadol, and mutually complements the analgesic effect of its NMDA receptor antagonism.[11]
Levorphanol shows a high rate of psychotomimetic side effects such as hallucinations and delirium, which have been attributed to its binding to and activation of the KOR.[12] At the same time however, activation of this receptor as well as of the DOR have been determined to contribute to its analgesic effects.[12]
Chemistry
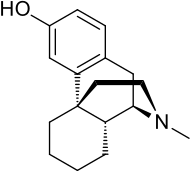
Chemically, levorphanol belongs to the morphinan class and is (−)-3-hydroxy-N-methyl-morphinan.[9] It is the "left-handed" (levorotatory) stereoisomer of racemorphan, the racemic mixture of the two stereoisomers with differing pharmacology. The "right-handed" (dextrorotatory) enantiomer of racemorphan is dextrorphan (DXO), an antitussive, potent dissociative hallucinogen (NMDA receptor antagonist), and weakly active opioid. DXO is an active metabolite of the pharmaceutical drug dextromethorphan (DXM), which, analogously to DXO, is an enantiomer of the racemic mixture racemethorphan along with levomethorphan, the latter of which has similar properties to those of levorphanol.
Society and culture
Name
Levorphanol is the INN, BAN, and DCF.[1][13][14] As the medically used tartrate salt, the drug is also known as levorphanol tartrate (USAN, BANM).[1][14] The former developmental code name of levorphanol at Roche was Ro 1-5431.[1][14]
Availability
As the tartrate salt, levorphanol is marketed by Sentynl Therapeutics and Virtus Pharmaceuticals in the U.S., and Canada under the brand name Levo-Dromoran.[13]
Legality
Levorphanol is listed under the Single Convention On Narcotic Drugs 1961 and is regulated like morphine in most countries. In the U.S., it is a Schedule II Narcotic controlled substance with a DEA ACSCN of 9220 and 2013 annual aggregate manufacturing quota of 4.5 kilos. The salts in use are the tartrate (free base conversion ratio 0.58) and hydrobromide (0.76).[15]
See also
- Cough syrup
- Racemorphan; Dextrorphan;
- Noscapine
- Codeine; Pholcodine
- Dextromethorphan; Dimemorfan
- Butamirate
- Pentoxyverine
- Tipepidine
- Cloperastine; Levocloperastine
References
- 1 2 3 4 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 656–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 8 9 10 11 "Levorphanol Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 22 November 2021.
- 1 2 Hogans, Beth B.; Barreveld, Antje M. (7 November 2019). Pain Care Essentials. Oxford University Press. p. PT164. ISBN 978-0-19-009142-2. Archived from the original on 11 December 2021. Retrieved 22 November 2021.
- ↑ "Levorphanol (Levo-Dromoran) Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 22 November 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 527. ISBN 9783527607495. Archived from the original on 2021-08-29. Retrieved 2021-10-21.
- 1 2 Gudin J, Fudin J, Nalamachu S (January 2016). "Levorphanol Use: Past, Present and Future". Postgraduate Medicine. 128 (1): 46–53. doi:10.1080/00325481.2016.1128308. PMID 26635068. S2CID 3912175.
- ↑ "Levorphanol Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 22 November 2021.
- ↑ Osborne NN (22 October 2013). Selected Topics from Neurochemistry. Elsevier Science. pp. 244–. ISBN 978-1-4832-8635-8.
- 1 2 3 Davis MP, Glare PA, Hardy J (2009) [2005]. Opioids in Cancer Pain (2nd ed.). Oxford, UK: Oxford University Press. ISBN 978-0-19-157532-7.
- ↑ Prommer E (March 2007). "Levorphanol: the forgotten opioid". Supportive Care in Cancer. 15 (3): 259–64. doi:10.1007/s00520-006-0146-2. PMID 17039381. S2CID 10916508.
- ↑ Nalamachu, S; Gudin, J (Apr 2016). "Levorphanol, another choice in opioid rotation". J Pain. 17: S14. doi:10.1016/j.jpain.2016.01.056.
- 1 2 Bruera ED, Portenoy RK (12 October 2009). Cancer Pain: Assessment and Management. Cambridge University Press. pp. 215–. ISBN 978-0-521-87927-9.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 606–. ISBN 978-3-88763-075-1. Archived from the original on 2017-04-01. Retrieved 2021-10-21.
- 1 2 3 Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Medi a. pp. 165–. ISBN 978-94-011-4439-1. Archived from the original on 1 April 2017. Retrieved 21 October 2021.
- ↑ "Conversion Factors for Controlled Substances". Diversion Control Division. U.S. Department of Justice • Drug Enforcement Administration. Archived from the original on 2019-12-07. Retrieved 2021-10-21.
External links
| Identifiers: |
|---|