GS-39783
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.558 |
| Chemical and physical data | |
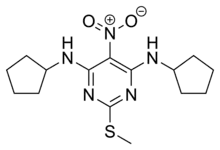
| Formula | C15H23N5O2S |
| Molar mass | 337.44 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
GS-39783 is a compound used in scientific research which acts as a positive allosteric modulator at the GABAB receptor.[1][2] It has been shown to produce anxiolytic effects in animal studies,[3][4][5] and reduces self-administration of alcohol,[6][7] cocaine[8][9][10] and nicotine.[11]
References
- ↑ Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, et al. (October 2003). "N,N'-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acidB receptor function". The Journal of Pharmacology and Experimental Therapeutics. 307 (1): 322–30. doi:10.1124/jpet.103.053074. PMID 12954816.
- ↑ Guery S, Floersheim P, Kaupmann K, Froestl W (November 2007). "Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABA B receptors". Bioorganic & Medicinal Chemistry Letters. 17 (22): 6206–11. doi:10.1016/j.bmcl.2007.09.023. PMC 2278029. PMID 17884493.
- ↑ Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF (June 2004). "Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior". Neuropsychopharmacology. 29 (6): 1050–62. doi:10.1038/sj.npp.1300413. PMID 15039762.
- ↑ Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. (September 2004). "Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines". The Journal of Pharmacology and Experimental Therapeutics. 310 (3): 952–63. doi:10.1124/jpet.104.066753. PMID 15113848.
- ↑ Jacobson LH, Cryan JF (April 2008). "Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents". Neuropharmacology. 54 (5): 854–62. doi:10.1016/j.neuropharm.2008.01.004. PMID 18328507.
- ↑ Orrù A, Lai P, Lobina C, Maccioni P, Piras P, Scanu L, et al. (November 2005). "Reducing effect of the positive allosteric modulators of the GABA(B) receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats". European Journal of Pharmacology. 525 (1–3): 105–11. doi:10.1016/j.ejphar.2005.10.005. PMID 16289452.
- ↑ Maccioni P, Pes D, Orrù A, Froestl W, Gessa GL, Carai MA, Colombo G (August 2007). "Reducing effect of the positive allosteric modulator of the GABA(B) receptor, GS39,783, on alcohol self-administration in alcohol-preferring rats". Psychopharmacology. 193 (2): 171–8. doi:10.1007/s00213-007-0776-1. PMID 17393141.
- ↑ Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DC (April 2004). "Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats". Psychopharmacology. 173 (1–2): 105–11. doi:10.1007/s00213-003-1706-5. PMID 14712341.
- ↑ Slattery DA, Markou A, Froestl W, Cryan JF (November 2005). "The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat". Neuropsychopharmacology. 30 (11): 2065–72. doi:10.1038/sj.npp.1300734. PMID 15841108.
- ↑ Lhuillier L, Mombereau C, Cryan JF, Kaupmann K (February 2007). "GABA(B) receptor-positive modulation decreases selective molecular and behavioral effects of cocaine". Neuropsychopharmacology. 32 (2): 388–98. doi:10.1038/sj.npp.1301102. PMC 1774586. PMID 16710312.
- ↑ Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A (July 2008). "Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats". The Journal of Pharmacology and Experimental Therapeutics. 326 (1): 306–14. doi:10.1124/jpet.108.139204. PMC 2574924. PMID 18445779.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.