Thiorphan
Further information: Racecadotril
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
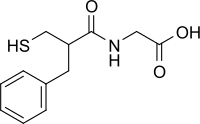
| Formula | C12H15NO3S |
| Molar mass | 253.32 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
Thiorphan is the active metabolite of the antidiarrheal racecadotril (acetorphan).[1] It prevents the degradation of endogenous enkephalins by acting as an enkephalinase inhibitor.[1][2]
References
- 1 2 Spillantini MG, Geppetti P, Fanciullacci M, Michelacci S, Lecomte JM, Sicuteri F (June 1986). "In vivo 'enkephalinase' inhibition by acetorphan in human plasma and CSF". European Journal of Pharmacology. 125 (1): 147–50. doi:10.1016/0014-2999(86)90094-4. PMID 3015640.
- ↑ Matheson AJ, Noble S (April 2000). "Racecadotril". Drugs. 59 (4): 829–35, discussion 836–7. doi:10.2165/00003495-200059040-00010. PMID 10804038.
| Rehydration | |
|---|---|
| Intestinal anti-infectives | |
| Intestinal adsorbents |
|
| Antipropulsives (opioids) |
|
| Intestinal anti-inflammatory agents |
|
| Antidiarrheal micro-organisms | |
| Other antidiarrheals | |
| |
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.