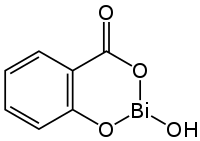
Bismuth subsalicylate
 | |
 | |
| Names | |
|---|---|
| Trade names | Pepto-Bismol, Peptic Relief, Pink Bismuth, others |
| Other names | Bismuth salts |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607040 |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C7H5BiO4 |
| Molar mass | 362.093 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Bismuth subsalicylate, sold under the brand name Pepto-Bismol among others, is an medication used for diarrhea and heartburn.[1] Its use for heartburn; however, is not generally recommended in the United Kingdom.[2] It is also used along with other medications to treat Helicobacter pylori infection.[1][2] It is taken by mouth either as a liquid or a tablet.[1][2]
Common side effects include a black tongue or dark stool.[1] Severe side effects may include Reye's syndrome and neurological problems.[1] It should not be used in people who are allergic to aspirin and in large doses may result in salicylate poisoning.[1][2] Use is the last third of pregnancy may harm the baby.[1] It works by forming a protective coating inside the stomach.[3]
Bismuth subsalicylate came into medical use in the 1910s.[3] It is available as a generic medication and over the counter.[4] While it is often described as an "antacid", it does not have antacid properties.[4] In the United States 30 tabs of 262 mg costs about 11 USD.[5]
Medical uses
Bismuth subsalicylate is used along with other medications to treat Helicobacter pylori infection.[1][2]
While it is used for heartburn, such use is not recommended in the United Kingdom due to side effects.[1][2] Evidence for benefit in heartburn without stomach ulcers is unclear.[1]
While it has been used for traveler's diarrhea, to be useful for this purpose requires high doses and therefore such use is controversial.[4]
Dosage
The typical dose in those over the age of 12 is 500 mg to 1 gram every 30 minutes to 6 hours to a maximum of 4 grams per day.[1][2] The liquid generally has 17.5 mg per 1 ml.[2]
When used together with antibiotics for H. pylori, a bismuth subsalicylate dose of two tablets of 262.4 mg four times per day for 14 days is typically used.[6]
Side effects

There are some side effects. It can cause a black tongue and black stools in some users of the drug when it combines with trace amounts of sulfur in saliva and the colon to form bismuth sulfide.[7] Bismuth sulfide is a highly insoluble black salt, and the discoloration seen is temporary and harmless.
Long-term use (greater than 6 weeks) may lead to accumulation and toxicity.[8] Some of the risks of salicylism can apply to the use of bismuth subsalicylate.[9][10][11]
Children should not take medication with bismuth subsalicylate while recovering from influenza or chicken pox, as there is an association between the use of salicylate-containing medications during certain viral infections and the onset of Reye syndrome.[12] For the same reason, it is typically recommended that nursing mothers not use medication containing bismuth subsalicylate because small amounts of the medication are excreted in human breast milk, and these pose a theoretical risk of Reye's syndrome to nursing children.[13]
The British National Formulary does not recommend bismuth-containing medications as an "antacid" (unless chelated), cautioning that absorbed bismuth can be neurotoxic, causing encephalopathy, and it can be constipating.[14]
Mechanism of action
The means by which it works is not well documented. It is thought to be some combination of the following:[15]
- Stimulation of absorption of fluids and electrolytes by the intestinal wall (antisecretory action)
- As a salicylate, reducing inflammation/irritation of stomach and intestinal lining through inhibition of prostaglandin G/H synthase 1/2
- Reduction in hypermotility of the stomach
- Binding of toxins produced by Escherichia coli
- Bactericidal action of a number of its subcomponents, including salicylic acid[16]
- Bactericidal action via a so-called oligodynamic effect in which small amounts of heavy metals such as bismuth damage many different bacteria species.
- It does not appear to have antacid properties[4]
In vitro and in vivo data have shown that bismuth subsalicylate hydrolyzes in the gut to bismuth oxychloride and salicylic acid and less commonly bismuth hydroxide. In the stomach, this is likely an acid-catalyzed hydrolysis. The salicylic acid is absorbed and therapeutical concentrations of salicylic acid can be found in blood after bismuth subsalicylate administration. Bismuth oxychloride and bismuth hydroxide are both believed to have bactericidal effects, as is salicylic acid for enterotoxigenic E. coli a common cause of "traveler's diarrhea."[16]
Organobismuth compounds have historically been used in growth media for selective isolation of microorganisms. Such salts have been shown to inhibit proliferation of Helicobacter pylori, other enteric bacteria, and some fungi.[17]
Chemistry

It has the chemical formula of C7H5BiO4,[18] and it is a colloidal substance obtained by hydrolysis of bismuth salicylate (Bi(C6H4(OH)CO2)3).
Bismuth subsalicylate is the only active ingredient in an over-the-counter drug that can leave a shiny metal oxide slag behind after being completely burnt with a blow torch.[19]
History
While bismuth salts were in use in Europe by the late 1700s, the combination of bismuth subsalicylate and zinc salts for astringency with salol (phenyl salicilate) appears to have begun in the US in the early 1900s as a remedy for life-threatening diarrhea in infants with cholera. At first sold directly to physicians, it was first marketed as Bismosal in 1918.[20]
Pepto-Bismol began being sold in 1900[20] or 1901[21] by a doctor in New York. It was originally sold as a remedy for infant diarrhea by Norwich Pharmacal Company under the name "Bismosal: Mixture Cholera Infantum".[20] It was renamed Pepto-Bismol in 1919. Norwich Eaton Pharmaceuticals was acquired by Procter and Gamble in 1982.[22]
As of 1946 and 1959, Canadian advertisements placed by Norwich show the product as Pepto-Besmal both in graphic and text.[23][24]
Pepto-Bismol is an over-the-counter drug currently produced by the Procter & Gamble company in the United States, Canada and the United Kingdom. Pepto-Bismol is made in chewable tablets[25] and swallowable caplets,[26] but it is best known for its original formula, which is a thick liquid. This original formula is a medium pink in color, with a teaberry (methyl salicylate) flavor.[27]
 Bottle of bismuth subsalicylate
Bottle of bismuth subsalicylate 1957 Life magazine ad for the product
1957 Life magazine ad for the product
Society and culture
Cost
In the United States 30 tabs of 262 mg costs about 11 USD.[5]
.svg.png.webp) Costs (US)
Costs (US).svg.png.webp) Prescriptions (US)
Prescriptions (US)
Other animals
Salicylates are very toxic to cats, and thus bismuth subsalicylate should not be administered to cats.[28]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Bismuth Salts Monograph for Professionals". Drugs.com. Archived from the original on 28 March 2016. Retrieved 8 October 2020.
- 1 2 3 4 5 6 7 8 BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 73. ISBN 9780857113658.
- 1 2 Sneader, Walter (2005). Drug Discovery: A History. John Wiley & Sons. p. 59. ISBN 978-0-470-01552-0. Archived from the original on 2021-08-28. Retrieved 2020-10-09.
- 1 2 3 4 Brodin, Michael (1998). The Over-The-Counter Drug Book. Simon and Schuster. p. 48. ISBN 978-0-671-01380-6. Archived from the original on 2021-08-28. Retrieved 2020-10-09.
- 1 2 "Bismuth subsalicylate Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 January 2021. Retrieved 9 October 2020.
- ↑ "Bismuth Subsalicylate, Metronidazole, And Tetracycline (Oral Route) Proper Use - Mayo Clinic". www.mayoclinic.org. Archived from the original on 14 October 2021. Retrieved 23 February 2022.
- ↑ "Why does Pepto-Bismol sometimes darken the tongue/stool and how long does it last?". Pepto-Bismol F/AQ. Pepto-Bismol. Archived from the original on 2015-07-11. Retrieved 2015-07-10.
- ↑ Gorbach SL (September 1990). "Bismuth therapy in gastrointestinal diseases". Gastroenterology. 99 (3): 863–75. doi:10.1016/0016-5085(90)90983-8. PMID 2199292.
- ↑ "Bismuth Subsalicylate". MedlinePlus. National Institutes of Health. Archived from the original on 2008-10-01. Retrieved 2016-05-18.
- ↑ Sainsbury SJ (December 1991). "Fatal salicylate toxicity from bismuth subsalicylate". The Western Journal of Medicine. 155 (6): 637–9. PMC 1003120. PMID 1812638.
- ↑ Vernace MA, Bellucci AG, Wilkes BM (September 1994). "Chronic salicylate toxicity due to consumption of over-the-counter bismuth subsalicylate". The American Journal of Medicine. 97 (3): 308–9. doi:10.1016/0002-9343(94)90017-5. PMID 8092182.
- ↑ Aspirin or Salicylate-Containing Medications Archived 2015-01-05 at the Wayback Machine, reyessyndrome.org
- ↑ "Food-borne and Waterborne Illness - Breastfeeding – CDC". cdc.gov. Archived from the original on 2017-07-29. Retrieved 2017-09-08.
- ↑ "1.1.1 Antacids and simeticone". Archived from the original on 2018-05-02. Retrieved 2018-06-17.
- ↑ Bismuth subsalicylate Archived 2012-03-06 at the Wayback Machine, DrugBank.
- 1 2 Sox TE, Olson CA (December 1989). "Binding and killing of bacteria by bismuth subsalicylate". Antimicrobial Agents and Chemotherapy. 33 (12): 2075–82. doi:10.1128/AAC.33.12.2075. PMC 172824. PMID 2694949.
- ↑ Dodge AG, Wackett LP (February 2005). "Metabolism of bismuth subsalicylate and intracellular accumulation of bismuth by Fusarium sp. strain BI". Applied and Environmental Microbiology. 71 (2): 876–82. doi:10.1128/AEM.71.2.876-882.2005. PMC 546758. PMID 15691943.
- ↑ Merck Index, 11th Edition, 1299
- ↑ Wesołowski, M. (1982). "Thermal decomposition of pharmaceutical preparations containing inorganic components". Microchimica Acta. Vienna. 77 (5–6): 451–464. doi:10.1007/BF01197125. S2CID 101586218.
- 1 2 3 Bierer DW (January–February 1990). "Bismuth subsalicylate: history, chemistry, and safety". Reviews of Infectious Diseases. 12 Suppl 1 (Supplement 1): S3-8. doi:10.1093/clinids/12.supplement_1.s3. JSTOR 4455445. PMID 2406853.
- ↑ "| History of Pepto Bismol". Archived from the original on 2019-03-06. Retrieved 2019-03-04.
- ↑ Davis, Dyer; et al. (May 1, 2004). Rising Tide: Lessons from 165 Years of Brand Building at Procter and Gamble. Harvard Business Press. p. 424. ISBN 9781591391470.
- ↑ "'Simple Diarrhoea' ad". Toronto Daily Star. August 16, 1946. p. 33.
- ↑ "'Pepto-Besmal puts out the fire of an upset stomach' ad". Toronto Daily Star. June 6, 1959.
- ↑ The trademark was extended to cover the tablets in 1973. Registration No. 0972198 Archived 2008-12-01 at the Wayback Machine, November 6, 1973.
- ↑ The capsules were introduced in 1983. Registration No. 1269605, March 13, 1984; cancelled July 16, 1990. http://tess2.uspto.gov/bin/showfield?f=doc&state=b8i462.2.1 Archived 2008-12-01 at the Wayback Machine.
- ↑ "Material Safety Data Sheet" (PDF). Procter & Gamble. Archived (PDF) from the original on 2017-12-09. Retrieved 2017-12-08.
- ↑ Cat Owner's Home Veterinary Handbook, Carlson and Giffin, page 390.
External links
| Identifiers: |
|---|
- Andrews PC, Deacon GB, Forsyth CM, Junk PC, Kumar I, Maguire M (August 2006). "Towards a structural understanding of the anti-ulcer and anti-gastritis drug bismuth subsalicylate". Angewandte Chemie. 45 (34): 5638–42. doi:10.1002/anie.200600469. PMID 16865763.