Naloxegol
 | |
| Names | |
|---|---|
| Trade names | Movantik, Moventig |
| Other names | PEGylated naloxol;[1] NKTR-118 |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | ~4.2% |
| Metabolism | Liver (CYP3A) |
| Elimination half-life | 6–11 h |
| Excretion | Feces (68%), urine (16%) |
| Chemical and physical data | |
| Formula | C34H53NO11 |
| Molar mass | 651.794 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Naloxegol, sold under the brand names Movantik and Moventig, is a medication used for opioid-induced constipation in people with long term non-cancer pain.[2] It may be used when laxatives are not effective.[3] It is taken by mouth.[2] It generally dose not affect pain management.[3]
Common side effects include abdominal pain, diarrhea, nausea, and headache.[2] Other side effects may include opioid withdrawal and gastrointestinal perforation.[2] Use in pregnancy has not shown harm; however such use has not been well studied.[4] Chemically it is naloxone attached to polyethylene glycol (PEF).[2] It is a peripherally acting μ-opioid receptor antagonist, which prevents opioids from binding to receptors in the gut.[2]
Naloxegol was approved for medical use in the United States and Europe in 2014.[2][5] In the United States it costs about 360 USD per month as of 2021.[6] This amount in the United Kingdom costs the NHS about £55.[3] As of 2015 it is no longer a controlled substance in the United States.[7]
Medical use
Naloxegol is indicated for the treatment of opioid-induced constipation (OIC) in patients with chronic non-cancer pain. It is recommended that any maintenance laxative be discontinued before starting naloxegol or be held for at-least 3 days. Naloxegol should be taken on an empty stomach at least two hours after the last meal.[8]
When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.[9]
Dosage
It is generally taken at a dose of 25 mg per day.[2] This was found safe and well tolerated for 52 weeks.[10]
Mechanism of action
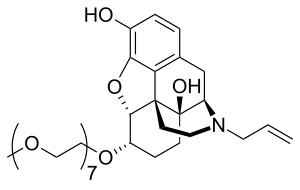
Chemically, naloxegol is a pegylated (polyethylene glycol-modified) derivative of α-naloxol. Specifically, the 6-α-hydroxyl group of α-naloxol is connected via an ether linkage to the free hydroxyl group of a monomethoxy-terminated n=7 oligomer of PEG, shown extending at the lower left of the molecule image at right. The "n=7" defines the number of two-carbon ethylenes, and so the chain length, of the attached PEG chain, and the "monomethoxy" indicates that the terminal hydroxyl group of the PEG is "capped" with a methyl group.[11] The pegylation of the 6-α-hydroxyl side chain of naloxol prevents the drug from crossing the blood-brain barrier (BBB).[9] As such, it can be considered the antithesis of the peripherally-acting opiate loperamide which is utilized as an opiate-targeting anti-diarrheal agent that does not cause traditional opiate side-effects due to its inability to accumulate in the central nervous system in normal subjects.
Pharmacodynamics
Naloxegol inhibits opioid binding in μ-opioid receptors in the gastrointestinal tract, thus decreasing the constipating effects (slowing of gastrointestinal motility and transit, hypertonicity, increased fluid reabsorption) associated with opioids.[12]
If naloxegol is coadministered with other opioid antagonists, there is a potential for additive effect and increased risk of opioid withdrawal.[8]
References
- ↑ Seifert R, Wieland T, Mannhold R, Kubinyi H, Folkers G (17 July 2006). G Protein-Coupled Receptors as Drug Targets: Analysis of Activation and Constitutive Activity. John Wiley & Sons. p. 227. ISBN 978-3-527-60695-5. Archived from the original on 28 June 2014. Retrieved 14 May 2012.
- 1 2 3 4 5 6 7 8 "Naloxegol Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 11 November 2021.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 71. ISBN 978-0857114105.
- ↑ "Naloxegol (Movantik) Use During Pregnancy". Drugs.com. Archived from the original on 27 January 2021. Retrieved 11 November 2021.
- ↑ "Moventig". Archived from the original on 27 February 2021. Retrieved 11 November 2021.
- ↑ "Naloxegol Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 31 October 2016. Retrieved 11 November 2021.
- ↑ "Schedules of Controlled Substances: Removal of Naloxegol From Control". www.deadiversion.usdoj.gov. Archived from the original on 2016-03-09. Retrieved 2016-02-27.
- 1 2 "Movantik prescribing information highlights". AstraZeneca. Archived from the original on 2019-05-02. Retrieved 2019-08-14.
- 1 2 Garnock-Jones KP (March 2015). "Naloxegol: a review of its use in patients with opioid-induced constipation". Drugs. 75 (4): 419–25. doi:10.1007/s40265-015-0357-2. PMID 25666542. S2CID 207488539.
- ↑ Webster L, Chey WD, Tack J, Lappalainen J, Diva U, Sostek M (October 2014). "Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation" (PDF). Alimentary Pharmacology & Therapeutics. 40 (7): 771–9. doi:10.1111/apt.12899. PMID 25112584. S2CID 34286557. Archived from the original on 2021-10-28. Retrieved 2021-10-24.
- ↑ Technically, the molecule that is attached via the ether link is O-methyl-heptaethylene glycol [that is, methoxyheptaethylene glycol, CH3OCH2CH2O(CH2CH2O)5CH2CH2OH], molecular weight 340.4, CAS number 4437-01-8. See Pubchem Staff (2016). "Compound Summary for CID 526555, Pubchem Compound 4437-01". PubChem Compound Database. Bethesda, MD, USA: NCBI, U.S. NLM. Archived from the original on 2016-02-05. Retrieved 28 January 2016.
- ↑ Garnock-Jones KP (March 2015). "Naloxegol: a review of its use in patients with opioid-induced constipation". Drugs. 75 (4): 419–25. doi:10.1007/s40265-015-0357-2. PMID 25666542. S2CID 207488539.
External links
| Identifiers: |
|---|