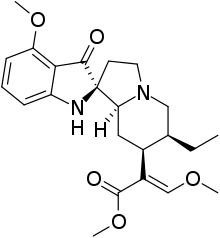
Mitragynine pseudoindoxyl
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl (2E)-2-[(1′S,6′S,7′S,8′aS)-6′-ethyl-4-methoxy-3-oxo-1,2′,3,3′,6′,7′,8′,8′a-octahydro-5′H-spiro[indole-2,1′-indolizin]-7′-yl]-3-methoxyprop-2-enoate | |
| Other names
Spiro[2H-indole-2,1'(5′H)-indolizine]-7'-acetic acid, 6'-ethyl-1,2',3,3',6',7',8',8'a-octahydro-4-methoxy-α-(methoxymethylene)-3-oxo-, methyl ester, (αE,2S,6′S,7′S,8′aS) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C23H30N2O5 |
| Molar mass | 414.502 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine.[1] It is an analgesic being more potent than morphine.[2][3]
Pharmacology
Mitragynine pseudoindoxyl is a μ opioid receptor agonist and δ opioid receptor antagonist and acts as a G protein biased agonist at μ opioid receptors and possesses a favourable side effect profile compared to conventional opioids.[4]
See also
- Mitraphylline
References
- ↑ Jansen KL, Prast CJ (1988). "Ethnopharmacology of kratom and the Mitragyna alkaloids". J Ethnopharmacol. 23 (1): 115–9. doi:10.1016/0378-8741(88)90121-3. PMID 3419199.
- ↑ Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S (April 2002). "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". J. Med. Chem. 45 (9): 1949–56. doi:10.1021/jm010576e. PMID 11960505.
- ↑ Yamamoto, L. T.; Horie, S.; Takayama, H.; Aimi, N.; Sakai, S.; Yano, S.; Shan, J.; Pang, P. K.; Ponglux, D.; Watanabe, K. (1999). "Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa". General Pharmacology. 33 (1): 73–81. doi:10.1016/S0306-3623(98)00265-1. PMID 10428019.
- ↑ Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan YX, Borics A, Pasternak GW, McLaughlin JP, Majumdar S (2016). "Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2". J. Med. Chem. 59 (18): 8381–97. doi:10.1021/acs.jmedchem.6b00748. PMC 5344672. PMID 27556704.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.