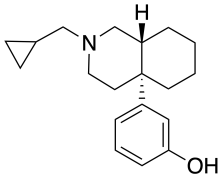
Ciprefadol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H27NO |
| Molar mass | 285.431 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ciprefadol is an opioid analgesic that is an isoquinoline derivative most closely related to cyclazocine and picenadol,[1] with a number of other related compounds known.[2][3][4][5] Ciprefadol is a mixed agonist–antagonist at μ-opioid receptors and can partly block the effects of morphine at low doses, though at higher doses it acts more like a full agonist. It is also a potent κ-opioid agonist, unlike the corresponding N-methyl and N-phenethyl derivatives which are reasonably μ-selective agonists.[6]
Synthesis
Fusion of an alicyclic ring onto the piperidine so as to form a perhydroisoquinoline is apparently consistent with analgesic activity.
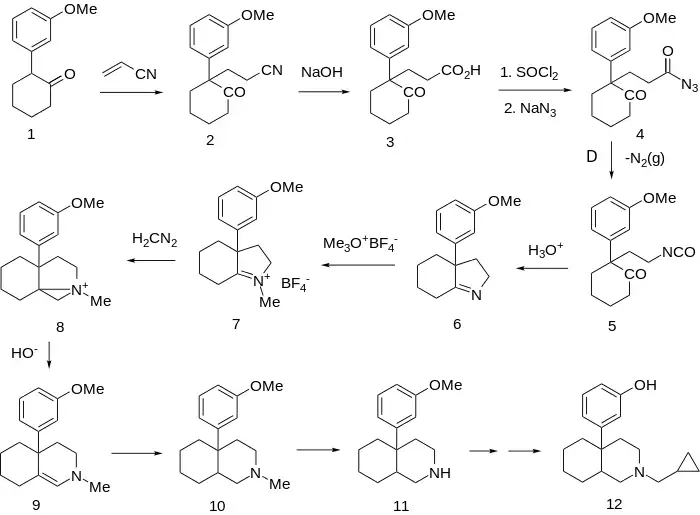
Synthesis of this agent, ciprefadol (12), starts with the Michael addition of the anion from cyclohexanone (1) onto acrylonitrile. Saponification of the nitrile (2) to the corresponding acid (3) followed by Curtius rearrangement leads to isocyanate (5). Acid hydrolysis of the isocyanate leads directly to the indoline (6), no doubt by way of internal Schiff base formation from the intermediate amine. Methylation by means of trimethyloxonium tetrafluoroborate affords ternary iminium salt 7. Treatment of that reactive carbonyl-like functionality with diazomethane gives the so-called azonia salt 8 (note the analogy to the hypothetical oxirane involved in ring expansion of ketones with diazomethane). Exposure of the aziridinium intermediate to base leads to ring opening and consequent formation of the octahydroisoquinoline (9). Reduction of the enamine (catalytic or borohydride) affords the perhydroisoquinoline 10. This compound is then subjected to one of the N-demethylation sequences and the resulting secondary amine alkylated with cyclopropylmethyl bromide; O-Demethylation of the phenol ether completes the preparation of ciprefadol (12).
References
- ↑ Dennis M. Zimmerman et al. N-cycloalkylmethyl decahydroisoquinolines. US Patent 4001248, Jun 7, 1974
- ↑ David R. Brittelli et al. 4a-Aryl-trans-decahydroisoquinolines. US Patent 4419517, Apr 8, 1975
- ↑ Henry Rapoport et al. Synthesis of 4A-aryl-decahydroisoquinolines. US Patent 4189583, Apr 26, 1978
- ↑ Judd DB, Brown DS, Lloyd JE, McElroy AB, Scopes DI, Birch PJ, et al. (January 1992). "Synthesis, antinociceptive activity, and opioid receptor profiles of substituted trans-3-(decahydro- and octahydro-4a-isoquinolinyl)phenols". Journal of Medicinal Chemistry. 35 (1): 48–56. doi:10.1021/jm00079a005. PMID 1310115.
- ↑ Carroll FI, Chaudhari S, Thomas JB, Mascarella SW, Gigstad KM, Deschamps J, Navarro HA (December 2005). "N-substituted cis-4a-(3-hydroxyphenyl)-8a-methyloctahydroisoquinolines are opioid receptor pure antagonists". Journal of Medicinal Chemistry. 48 (26): 8182–93. doi:10.1021/jm058261c. PMC 2585695. PMID 16366600.
- ↑ Zimmerman DM, Cantrell BE, Swartzendruber JK, Jones ND, Mendelsohn LG, Leander JD, Nickander RC (March 1988). "Synthesis and analgesic properties of N-substituted trans-4a-aryldecahydroisoquinolines". Journal of Medicinal Chemistry. 31 (3): 555–60. doi:10.1021/jm00398a011. PMID 2831363.