Sunobinop
 | |
| Clinical data | |
|---|---|
| Other names | IMB-115 |
| Drug class | Nociceptin receptor agonist |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
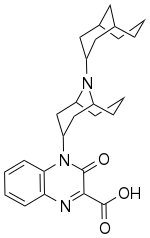
| Formula | C26H33N3O3 |
| Molar mass | 435.568 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sunobinop (developmental code name IMB-115) is a nociceptin receptor partial agonist and opioid receptor pan-antagonist.[1] As of March 2022, it is under investigation for the treatment of insomnia, fibromyalgia, and overactive bladder.[1][2]
See also
- List of investigational sleep drugs § Nociceptin receptor agonists
- Nociceptin receptor
References
- 1 2 "Sunobinop - Shionogi/Imbrium Therapeutics". AdisInsight. Springer Nature Switzerland AG.
- ↑ WO application 2018020418, Harris SC, Kapil RP, Kyle DJ, Whiteside G, "Treatment and prevention of sleep disorders", published 2018-02-01, assigned to Purdue Pharma L.P.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.