Oxilorphan
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.664 |
| Chemical and physical data | |
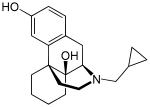
| Formula | C20H27NO2 |
| Molar mass | 313.441 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Oxilorphan (INN, USAN) (developmental code name L-BC-2605) is an opioid antagonist of the morphinan family that was never marketed.[1] It acts as a μ-opioid receptor (MOR) antagonist but a κ-opioid receptor (KOR) partial agonist, and has similar effects to naloxone and around the same potency as an MOR antagonist.[2] Oxilorphan has some weak partial agonist actions at the MOR (with miosis, nausea, dizziness, and some euphoria observed)[3][4] and can produce hallucinogenic/dissociative effects at sufficient doses, indicative of KOR activation.[5] It was trialed for the treatment of opioid addiction, but was not developed commercially.[6] The KOR agonist effects of oxilorphan are associated with dysphoria, which combined with its hallucinogenic effects, serve to limit its clinical usefulness; indeed, many patients who experienced these side effects refused to take additional doses in clinical trials.[7]
See also
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 916–. ISBN 978-1-4757-2085-3.
- ↑ Pircio AW, Gylys JA (April 1975). "Oxilorphan (l-N-cyclopropylmethyl-3,14-dihydroxymorphinan): a new synthetic narcotic antagonist". The Journal of Pharmacology and Experimental Therapeutics. 193 (1): 23–34. PMID 237112.
- ↑ Sellers EM, Thakur R (April 1976). "Partial agonist properties and toxicity of oral oxilorphan". Journal of Clinical Pharmacology. 16 (4): 183–7. doi:10.1002/j.1552-4604.1976.tb01515.x. PMID 4472. S2CID 2819499.
- ↑ Gordon M (22 November 1974). "Abuse of CNS Agents". Annual Reports in Medicinal Chemistry. Vol. 9. Academic Press. pp. 41–. ISBN 978-0-08-058353-2.
- ↑ Leander JD (January 1983). "Evidence that nalorphine, butorphanol and oxilorphan are partial agonists at a kappa-opioid receptor". European Journal of Pharmacology. 86 (3–4): 467–70. doi:10.1016/0014-2999(83)90198-x. PMID 6131829.
- ↑ Tennant FS, Tate JA, Ruckel E (June 1976). "Clinical trial in post-addicts with oxilorphan (levo-BC-2605): a new narcotic antagonist". Drug and Alcohol Dependence. 1 (5): 329–37. doi:10.1016/0376-8716(76)90035-1. PMID 13984.
- ↑ National Research Council (U.S.). Committee on Problems of Drug Dependence (1975). Problems of drug dependence. National Academy of Sciences. ISBN 9780309024174.