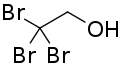
Tribromoethanol
 | |
| Clinical data | |
|---|---|
| Trade names | Avertin |
| Other names | Tribromoethyl alcohol |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.822 |
| Chemical and physical data | |
| Formula | C2H3Br3O |
| Molar mass | 282.757 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 73–79 °C (163–174 °F) [1][2] |
| Boiling point | 92–93 °C (198–199 °F) at 10 mmHg[1] |
SMILES
| |
InChI
| |
2,2,2-Tribromoethanol, often called just tribromoethanol, is a chemical compound with formula Br3C−CH2OH. Its molecule can be described as that of ethanol, with the three hydrogen atoms in position 2 (on the methyl group) replaced by bromine. It is a white crystalline solid, soluble in water and other solvents, that absorbs strongly in the UV below 290 nm.[2]
Tribromoethanol is used in medicine and biology as an anesthetic, and has been available commercially for that purpose by the trade name Avertin. It was formerly used on humans[3] and is still often used on laboratory animals,[4] and to capture wild birds.[5] It is also used in plastics industry as a polymerization initiator.[6][7]
Uses
Animal anesthetic
Tribromoethanol is often used to anesthetize laboratory animals, particularly rodents, before surgery.[4] As a solution in tert-amyl alcohol, it has the brand name Avertin.[8] The tert-amyl alcohol acts as a weak hypnotic, in addition to improving the solubility of the tribromoethanol. Administered intravenously, tribromoethanol (Avertin) causes rapid and deep anesthesia followed by rapid and full postoperative recovery in small mammals.[9] Recently its safety for this purpose has been questioned.[10]
Wildlife capture
Tribromoethanol has also been long used as spiked grain bait to capture wild turkeys for research and wildlife management purposes.[5] However, the birds learn to avoid it, for over a year, after a single exposure, and will drive other flock members away from the bait when they detect it.[11]
Human anesthetic
In the first half of the 20th century, Avertin was also used in humans as a general anesthetic or basal narcotic to induce unconsciousness prior to the administration of other anesthetic agents. It was administered rectally as a retention enema or by intravenous injection. Its rectal use was particularly favored for pediatrics, head or neck surgery, or in mentally unstable or anxious patients.[3] Electrophysiology studies showed that tribromoethanol acts as a positive allosteric modulator of the inhibitory GABAA and glycine receptors, a mechanism similar to that seen with the related compound 2,2,2-trichloroethanol.[12] Bromal hydrate (2,2,2-tribromoethanol-1,1-diol), a compound also recognized to produce general anesthesia in animals, is metabolized to tribromoethanol.[13]
Polymerization initiator
Tribromoethanol can be used as a functional polymerization initiator for the introduction of α-hydroxyl groups in poly(methyl methacrylate) (PMMA) and poly(n-butyl acrylate) (PBAK).[6][14][7]
Chemistry
Photolysis
Tribromoethanol should be kept refrigerated and in the dark, to prevent decomposition by light into hydrobromic acid and possibly 2,2-dibromoacetaldehyde or glycolic acid.[15][2]
See also
References
- 1 2 "2,2,2-Tribromoethanol". Sigma-Aldrich.
- 1 2 3 V. G. Sorensen, V. M. Bhale, K. J. McCallum, and R. J. Woods (1970): "Radiolysis of aqueous 2,2,2-tribromoethanol solutions". Canadian Journal of Chemistry, volume 48, issue 16, pages 2542-2548. doi:10.1139/v70-430
- 1 2 Edwards G (December 1945). "Tribromethyl alcohol (avertin, bromethol), 1928-1945". Proceedings of the Royal Society of Medicine. 39 (2): 71–6. doi:10.1177/003591574503900203. PMID 21010258.
- 1 2 "Tribromoethanol (Avertin)". Cold Spring Harbor Protocols. Cold Spring Harbor Laboratory. 2006: pdb.rec701. 2006. doi:10.1101/pdb.rec701.
- 1 2 Ronnie R. Evans, John W. Goertz and Clifford T. Williams (1975): "Capturing wild turkeys with tribromoethanol". Journal of Wildlife Management, volume 39, issue 3, pages 630-634. doi:10.2307/3800410 JSTOR 3800410
- 1 2 G. Moineau, M. Minet, Ph. Dubois, Ph. Teyssié, T. Senninger, and R. Jérôme (1999): "Controlled radical polymerization of (meth)acrylates by ATRP with NiBr2(PPh3)2 as catalyst". Macromolecules, volume 32, issue 1, pages 27–35. doi:10.1021/ma980995u
- 1 2 Roniérik P. Vieira, Andréia Ossig, Janaína M. Perez, Vinícius G. Grassi, Cesar L. Petzhold, Augusto C. Peres, João M. Costa, and Liliane M. F. Lona (2015): "Styrene ATRP using the new initiator 2,2,2‐tribromoethanol: Experimental and simulation approach". Polymer Engineering & Science, volume 55, issue 10. doi:10.1002/pen.24113
- ↑ "Guidelines for the Use of Tribromoethanol/Avertin Anesthesia" (PDF). National Cancer Institute.
- ↑ Weiss J, Zimmermann F (April 1999). "Tribromoethanol (Avertin) as an anaesthetic in mice". Laboratory Animals. 33 (2): 192–3. doi:10.1258/002367799780578417. PMID 10780824.
- ↑ Robert E. Meyer and Richard E. Fish (2005) "A review of tribromoethanol anesthesia for production of genetically engineered mice and rats". Lab Animal, volume 34, pages 47–52. doi:10.1038/laban1105-47
- ↑ J. Rickie Davis, David C. Guynn, Jr. and Bryan D. Hyder (1994): "Feasibility of using tribromoethanol to recapture wild turkeys". Wildlife Society Bulletin, volume 22, issue 3, pages 496-500. JSTOR 3783394
- ↑ Krasowski MD, Harrison NL (February 2000). "The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations". British Journal of Pharmacology. 129 (4): 731–43. doi:10.1038/sj.bjp.0703087. PMC 1571881. PMID 10683198.
- ↑ Lehmann G, Knoefel PK (August 1938). "Trichlorethanol, tribromethanol, chloral hydrate and bromal hydrate". Journal of Pharmacology and Experimental Therapeutics. 63 (4): 453–65.
- ↑ Mohammed Dirany, Marylène Vayer, Christophe Sinturel, René Erre, Patrick Lacroix-Desmazes, and Bernard Boutevin (2009): "Synthesis and characterization of polystyrene-block-polylactide by combination of ATRP and ROP using tribromoethanol as initiator: Precursors to ordered nanoporous materials". Online article at the HAL website. Accessed on 2020-07-11.
- ↑ T. Nicol, B. Vernon-Roberts, and D. C. Quantock (1965): "Protective effect of oestrogens against the toxic decomposition products of tribromoethanol". Nature, volume 208, pages 1098–1099. doi:10.1038/2081098a0