Thiamylal
 | |
| Clinical data | |
|---|---|
| Other names | Thiamylal, Thioseconal, Surital |
| AHFS/Drugs.com | International Drug Names |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 14.3 h (cats) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.927 |
| Chemical and physical data | |
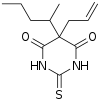
| Formula | C12H18N2O2S |
| Molar mass | 254.35 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Thiamylal (Surital) is a barbiturate derivative invented in the 1950s. It has sedative, anticonvulsant, and hypnotic effects, and is used as a strong but short acting sedative. Thiamylal is still in current use, primarily for induction in surgical anaesthesia[1] or as an anticonvulsant to counteract side effects from other anaesthetics.[2] It is the thiobarbiturate analogue of secobarbital.
References
- ↑ Hsieh MY, Hung GY, Hsieh YL, Chang CY, Hwang B (2005). "Deep sedation with methohexital or thiamylal with midazolam for invasive procedures in children with acute lymphoblastic leukemia". Acta Paediatrica Taiwanica = Taiwan Er Ke Yi Xue Hui Za Zhi. 46 (5): 294–300. PMID 16640004.
- ↑ Tsai CJ, Wang HM, Lu IC, Tai CF, Wang LF, Soo LY, Lu DV (February 2007). "Seizure after local anesthesia for nasopharyngeal angiofibroma". The Kaohsiung Journal of Medical Sciences. 23 (2): 97–100. doi:10.1016/S1607-551X(09)70383-3. PMID 17339174.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.