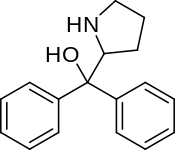
Diphenylprolinol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.118.791 |
| Chemical and physical data | |
| Formula | C17H19NO |
| Molar mass | 253.345 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Diphenylprolinol (D2PM), or (R/S)-(±)-diphenyl-2-pyrrolidinyl-methanol, is a norepinephrine-dopamine reuptake inhibitor which is used as a designer drug.[1][2]
Pharmacology
The dextrorotary (R)-(+)-enantiomer is the more pharmacologically active, although a variety of related derivatives have been studied.[3]
Side effects including chest pain (suggestive of possible cardiovascular toxicity) have been seen following recreational use of diphenylprolinol, although it was combined with glaucine in a party pill product, thus making it impossible to say for certain which drug was responsible.[4]
Other uses
Diphenylprolinol can be used to prepare the chiral CBS catalyst, which is used for enantioselective organic synthesis.[5]
See also
- 2-Diphenylmethylpyrrolidine (Desoxy-diphenylprolinol)
- Desoxypipradrol
- Pipradrol
- Prolinol
- Corey-Bakshi-Shibata reduction
References
- ↑ Wood DM, et al. (2008). "Detection of the novel recreational drug Diphenyl-2-pyrrolidinemethanol (D2PM) sold legally in combination with 1-Benzylpiperzaine (BZP)". Clinical Toxicology. 46 (5): 393. doi:10.1080/15563650802071703. PMID 18568796.
- ↑ Davies S. Drug Trends and New Designer Drugs. St George's University of London. 6 November 2008.
- ↑ US patent 5925666, Paul F. Jackson et al., "Pharmaceutical compositions and methods for treating compulsive disorders using pyrrolidine derivatives"
- ↑ Lidder, S; Dargan, P; Sexton, M; Button, J; Ramsey, J; Holt, D; Wood, D (2008). "Cardiovascular toxicity associated with recreational use of diphenylprolinol (diphenyl-2-pyrrolidinemethanol D2PM)". Journal of Medical Toxicology. 4 (3): 167–9. doi:10.1007/bf03161195. PMC 3550040. PMID 18821489.
- ↑ Corey, E. J.; Bakshi, R. K.; Shibata S. (1987). "Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines Mechanism and synthetic implications". J. Am. Chem. Soc. 109 (18): 5551–5553. doi:10.1021/ja00252a056. ISSN 0002-7863.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.