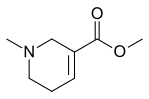
Arecoline
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.514 |
| Chemical and physical data | |
| Formula | C8H13NO2 |
| Molar mass | 155.197 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.0495 g/cm3 |
| Melting point | 27 °C (81 °F) |
| Boiling point | 209 °C (408 °F) |
SMILES
| |
InChI
| |
| | |
Arecoline (/əˈrɛkəliːn/) is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm (Areca catechu).[2] It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy, euphoria and relaxation. Its psychoactive effects are comparable to that of nicotine.
Chemistry
Arecoline is a base, and its conjugate acid has a pKa ~ 6.8.[3] Arecoline is volatile in steam, miscible with most organic solvents and water, but extractable from water by ether in presence of dissolved salts. Being basic, arecoline forms salts with acids. The salts are crystalline, but usually deliquescent: the hydrochloride, arecoline•HCl, forms needles, m.p. 158 °C;[3] the hydrobromide, arecoline•HBr, forms slender prisms, mp. 177–179 °C from hot alcohol; the aurichloride, arecoline•HAuCl4, is an oil, but the platinichloride, arecoline2•H2PtCl6, mp. 176 °C, crystallizes from water in orange-red rhombohedrons. The methiodide forms glancing prisms, mp. 173-174 °C.
Pharmacology
Arecoline is the primary active ingredient responsible for the central nervous system effects of the areca nut. Arecoline has been compared to nicotine; however, nicotine agonizes nicotinic acetylcholine receptors, whereas arecoline is primarily a partial agonist of muscarinic acetylcholine receptors[4][5], leading to its parasympathetic effects. In frogs, arecoline also acts as an antagonist (or very weak partial agonist) at α4 and α6-containing nicotinic acetylcholine receptors and as a silent antagonist at α7 nicotinic receptors, which may account for its anti-inflammatory activity.[6] Arecoline also inhibits AMPK through generation of ROS in several types of cells.[7]
Effects on nervous system
Arecoline promotes excitation and decreases sleeping time. It also enhances learning and memory. Intraperitoneal administration of arecoline decreases locomotor activity dose-dependently. Arecoline reversed scopolamine induced memory loss. It could also decrease symptoms of depression and schizophrenia [8]
Effects on cardiovascular system
AN (Areca Nut) is a vasodilator mainly due to the presence of arecoline. It also has anti-thrombosis and anti-atherogenic effects by increasing plasma nitric oxide, eNos, and mRNA expression and decreasing IL-8 along with other downregulations.[8]
Effects on endocrine system
It increases the level of testosterone by stimulating Leydig's cells as well as levels of FSH and LH.[9][10] It also activates HPA axis and stimulates CRH release. It prevents the dysfunction of B cells of the pancreas from high fructose intake.[8]
Effects on digestive system
Arecoline has the ability to stimulate the digestive system through the activation of musacarinic receptors. Areca nut water extract could increase the contractions of gastric smooth muscle and muscle strips of the duodenum, ileum, and colon significantly. This activity could be caused by arecoline.[8]
Pharmacokinetic
Arecoline is metabolized by both kidneys and liver.[11] Currently, 11 metabolites of arecoline are documented among which N-methylnipecotic acid was found to be a major metabolite of both arecoline and arecaidine.[12] Lime is said to hydrolyse almost all arecoline to arecaidine, a GABA reuptake inhibitor.[13] Arecaidine is also formed during liver metabolism of arecoline in rats.[12]
Uses
Owing to its muscarinic and nicotinic agonist properties, arecoline has shown improvement in the learning ability of healthy volunteers. Since one of the hallmarks of Alzheimer's disease is a cognitive decline, arecoline was suggested as a treatment to slow down this process and arecoline administered intravenously did indeed show modest verbal and spatial memory improvement in Alzheimer's patients, though due to arecoline's possible carcinogenic properties,[14] it is not the first drug of choice for this degenerative disease.[15] In many Asian cultures, the areca nut is chewed along with betel leaf to obtain a stimulating effect.[16]
Arecoline has also been used medicinally as an antihelmintic (a drug against parasitic worms).[17] Arecoline also increase testosterone in low doses [9]
Toxicity
LD50: 100 mg/kg, administered subcutaneously in mouse.[3] Also, the minimum lethal dose (MLD) values of arecoline in mice, dog and horse is 100 mg/kg, 5 mg/kg and 1.4 mg/kg respectively. It causes Oral Submucous Fibrosis by stimulating collagen, interleukin 6, keratinocyte growth factor-1, IGF-1, cystatin C, tissue inhibitor of matrix metalloproteinases in the mouth. Current science is confident that areca nut chewing is carcinogenic. Research suggests this is probably at least partly because of arecoline itself, although it could also be from the other constituents of the nut as well, some of which are precursors to nitrosamines that form in the mouth during chewing. Section 5.5 Evaluation on page 238 of IARC Monograph 85-6 states the following:[18]
- [...]
- There is sufficient evidence in humans for the carcinogenicity of betel quid without tobacco. Betel quid without tobacco causes oral cancer.
- There is sufficient evidence in experimental animals for the carcinogenicity of betel quid without tobacco.
- There is sufficient evidence in experimental animals for the carcinogenicity of betel quid with tobacco.
- There is sufficient evidence in experimental animals for the carcinogenicity of areca nut.
- There is sufficient evidence in experimental animals for the carcinogenicity of areca nut with tobacco.
- There is limited evidence in experimental animals for the carcinogenicity of arecoline.
- There is inadequate evidence in experimental animals for the carcinogenicity of arecaidine.
- [...]
See also
- Chavibetol
- Muscarine
References
- ↑ "Poisons Standard October 2020".
- ↑ Ghelardini C, Galeotti N, Lelli C, Bartolini A (2001). "Arecoline M1 receptor activation is a requirement for arecoline analgesia". Il Farmaco. 56 (5–7): 383–5. doi:10.1016/S0014-827X(01)01091-6. hdl:2158/327019. PMID 11482763.
- 1 2 3 The Merck Index, 10th Ed. (1983) p.113, Rahway: Merck & Co.
- ↑ "Differential Receptor Occupancy requirements for Muscarinic Cholinergic Stimulation of Inositol Lipid Hydrolysis in Brain and in Neuroblastomas". citeseerx.ist.psu.edu. Archived from the original on 2022-02-15. Retrieved 2022-02-15.
- ↑ Mei, Lin (1990). "Pharmacologic Comparison of Selected Agonists for the M1 Muscarinic Receptor in Transfected Murine Fibroblast Cells (B82)" (PDF).
- ↑ Papke, Roger L.; Horenstein, Nicole A.; Stokes, Clare (2015). "Nicotinic Activity of Arecoline, the Psychoactive Element of "Betel Nuts", Suggests a Basis for Habitual Use and Anti-Inflammatory Activity". PLOS ONE. 10 (10): e0140907. Bibcode:2015PLoSO..1040907P. doi:10.1371/journal.pone.0140907. PMC 4619380. PMID 26488401. S2CID 7207479.
- ↑ Yen CY, Lin MH, Liu SY, Chiang WF, Hsieh WF, Cheng YC, Hsu KC, Liu YC (May 2011). "Arecoline-mediated inhibition of AMP-activated protein kinase through reactive oxygen species is required for apoptosis induction". Oral Oncology. 47 (5): 345–51. doi:10.1016/j.oraloncology.2011.02.014. PMID 21440488.
- 1 2 3 4 Liu, Yu-Jie; Peng, Wei; Hu, Mei-Bian; Xu, Min; Wu, Chun-Jie (2016). "The pharmacology, toxicology and potential applications of arecoline: A review". Pharmaceutical Biology. 54 (11): 2753–2760. doi:10.3109/13880209.2016.1160251. PMID 27046150. S2CID 43564006.
- 1 2 Wang, S. W.; Hwang, G. S.; Chen, T. J.; Wang, P. S. (2008). "Effects of arecoline on testosterone release in rats". American Journal of Physiology. Endocrinology and Metabolism. 295 (2): E497-504. doi:10.1152/ajpendo.00045.2008. PMID 18559981.
- ↑ Saha, Indraneel; Das, Joydeep; Maiti, Biswaranjan; Chatterji, Urmi (2015). "A Protective Role of Arecoline Hydrobromide in Experimentally Induced Male Diabetic Rats". BioMed Research International. 2015: 1–12. doi:10.1155/2015/136738. PMC 4324734. PMID 25695047.
- ↑ "Arecoline - an overview | ScienceDirect Topics". Archived from the original on 2020-11-27.
- 1 2 Giri S, Idle JR, Chen C, Zabriskie TM, Krausz KW, Gonzalez FJ (June 2006). "A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse". Chemical Research in Toxicology. 19 (6): 818–27. doi:10.1021/tx0600402. PMC 1482804. PMID 16780361.
- ↑ Johnston, G. A. R.; Krogsgaard-Larsen, P.; Stephanson, A. (1975). "Betel nut constituents as inhibitors of γ-aminobutyric acid uptake". Nature. 258 (5536): 627–628. Bibcode:1975Natur.258..627J. doi:10.1038/258627a0. ISSN 0028-0836. PMID 1207742. S2CID 4147760.
- ↑ Saikia JR, Schneeweiss FH, Sharan RN (1999). "Arecoline-induced changes of poly-ADP-ribosylation of cellular proteins and its influence on chromatin organization". Cancer Letters. 139 (1): 59–65. doi:10.1016/S0304-3835(99)00008-7. PMID 10408909.
- ↑ Christie JE, Shering A, Ferguson J (1981). "Physostigmine and arecoline: effects of intravenous infusions in Alzheimer's presenile dementia". British Journal of Psychiatry. 138 (1): 46–50. doi:10.1192/bjp.138.1.46. PMID 7023592.
- ↑ Gupta Prakash Chandra; Ray Cecily S (July 2004). "Epidemiology of betel quid usage" (PDF). Ann. Acad. Med. Singap. 33 (4 Suppl): 31–6. PMID 15389304. Archived from the original (PDF) on 2009-06-12.
- ↑ Yusuf H, Yong SL (2002). "Oral submucous fibrosis in a 12-year-old Bangladeshi boy: a case report and review of literature". International Journal of Paediatric Dentistry. 12 (4): 271–6. doi:10.1046/j.1365-263X.2002.00373.x. PMID 12121538.
- ↑ International Agency for Research on Cancer (2005). Betel-quid and areca-nut chewing. IARC Monograph 85-6 (PDF). IARC. ISBN 978-92-832-1285-0.