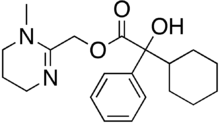
Oxyphencyclimine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.313 |
| Chemical and physical data | |
| Formula | C20H28N2O3 |
| Molar mass | 344.455 g·mol−1 |
InChI
| |
| | |
Oxyphencyclimine is a muscarinic receptor antagonist, given orally to treat peptic ulcer disease and gastrointestinal spasms. It has antispasmodic and antimotility properties.
Synthesis
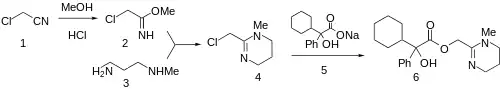
The reaction of chloroacetonitrile (1) with methanol and hydrogen chloride leads to the corresponding iminoether (Pinner reaction). Condensation of 2 with 3-methylaminopropylamine gives (3) gives the corresponding tetrahydropyrimidine (4). Displacement of the halogen with the sodium salt 5 affords oxyphencyclimine (6).
References
- ↑ Faust JA, Mori A, Sahyun M (1959). "Antispasmodics: Esters of Heterocyclic Alcohols". Journal of the American Chemical Society. 81 (9): 2214. doi:10.1021/ja01518a051.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.