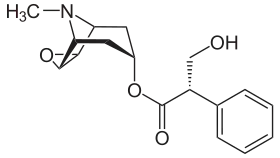
Hyoscine
 | |
 | |
| Names | |
|---|---|
| Trade names | Transdermscop, Kwells, others |
| Other names | Scopolamine, hyoscine hydrobromide, scopolamine hydrobromide[1] |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | by mouth, skin patch, eye drops, subcutaneous, intravenous, sublingual, rectal, buccal transmucosal, intramuscular |
| Defined daily dose | 0.9 mg (by mouth) 0.9 mg (parenteral)[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 4.5 hours[3] |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C17H21NO4 |
| Molar mass | 303.358 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Hyoscine, also known as scopolamine,[4] is a medication used to treat motion sickness and postoperative nausea and vomiting.[1] It is also sometimes used before surgery to decrease saliva.[1] When used by injection, effects begin after about 20 minutes and last for up to 8 hours.[1] It may also be used by mouth and as a skin patch.[1]
Common side effects include sleepiness, blurred vision, dilated pupils, and dry mouth.[1] It is not recommended in people with angle-closure glaucoma or bowel obstruction.[1] It is unclear if use during pregnancy is safe; however, it appears to be safe during breastfeeding.[5] Hyoscine is in the antimuscarinic family of medications and works by blocking some of the effects of acetylcholine within the nervous system.[1]
Hyoscine was first written about in 1881 and started to be used for anesthesia around 1900.[6][7] It is on the World Health Organization's List of Essential Medicines.[8] Hyoscine is the main active component produced by certain plants of the nightshade family which historically have been used as psychoactive drugs.[9] The name "scopolamine" is derived from one type of nightshade known as Scopolia while the name "hyoscine" is derived from another type known as Hyoscyamus niger.[10][11]
Medical use
Hyoscine has a number of uses in medicine, where it is used to treat the following:[12][13]
- Postoperative nausea and vomiting and sea sickness, leading to its use by scuba divers[14][15]
- Motion sickness (where it is often applied as a transdermal patch behind the ear)
- Gastrointestinal spasms
- Renal or biliary spasms
- Aid in gastrointestinal radiology and endoscopy
- Irritable bowel syndrome
- Clozapine-induced hypersalivation (drooling)
- Bowel colic
- Eye inflammation[16]
It is sometimes used as a premedication, (especially to reduce respiratory tract secretions) in surgery, mostly commonly by injection.[12][13]
Breastfeeding
Hyoscine enters breast milk by secretion. Although no human studies exist to document the safety of hyoscine while nursing, the manufacturer recommends that caution be taken if hyoscine is administered to a breastfeeding woman.[17]
Elderly
The likelihood of experiencing adverse effects from hyoscine is increased in the elderly relative to younger people. This phenomenon is especially true for older people who are also on several other medications. It is recommended that hyoscine use should be avoided in this age group because of these potent anticholinergic adverse effects which have also been linked to an increased risk for dementia.[18][19]
Dosage
The defined daily dose is 0.9 mg (by mouth) or 0.9 mg (parenteral)[2]
Hyoscine can be taken by mouth, subcutaneously, ophthalmically and intravenously, as well as via a transdermal patch.[20] The transdermal patch (e.g., Transderm Scōp) for prevention of nausea and motion sickness employs hyoscine base, and is effective for up to three days.[21] The oral, ophthalmic, and intravenous forms have shorter half-lives and are usually found in the form hyoscine hydrobromide (for example in Scopace, soluble tablets or Donnatal).
NASA is currently developing a nasal administration method. With a precise dosage, the NASA spray formulation has been shown to work faster and more reliably than the oral form.[22]
Side effects
Side effect incidence:[23][24][25][26]
Uncommon (0.1–1% incidence):
- Dry mouth
- Dyshidrosis (reduced ability to sweat in order to cool off)
- Tachycardia (usually occurs at higher doses and is succeeded by bradycardia)
- Bradycardia
- Urticaria (hives)
- Pruritus (itching)
Rare (<0.1% incidence):
- Constipation
- Urinary retention
- Hallucinations
- Agitation
- Confusion
- Restlessness
- Seizures
Unknown frequency:
- Anaphylactic shock
- Anaphylactic reactions
- Dyspnea (shortness of breath)
- Rash
- Erythema
- Other hypersensitivity reactions
- Blurred vision
- Mydriasis (dilated pupils)
- Drowsiness
- Dizziness
- Somnolence
Overdose
Physostigmine is a cholinergic drug that readily crosses the blood-brain barrier, and has been used as an antidote to treat the central nervous system depression symptoms of a hyoscine overdose.[27] Other than this supportive treatment, gastric lavage and induced emesis (vomiting) are usually recommended as treatments for oral overdoses.[26] The symptoms of overdose include:[25][26]
- Tachycardia
- Arrhythmia
- Blurred vision
- Photophobia
- Urinary retention
- Drowsiness or paradoxical reaction which can present with hallucinations
- Cheyne-Stokes respiration
- Dry mouth
- Skin reddening
- Inhibition of gastrointestinal motility
Interactions
Due to interactions with metabolism of other drugs, hyoscine can cause significant unwanted side effects when taken with other medications. Specific attention should be paid to other medications in the same pharmacologic class as hyoscine, also known as anticholinergics. The following medications could potentially interact with the metabolism of hyoscine: analgesics/pain medications, ethanol, zolpidem, thiazide diuretics, buprenorphine, anticholinergic drugs such as tiotropium, etc.
Pharmacodynamics
The muscarinic antagonism of scopolamine remains the standard method for inducing cognitive deficits in animals and in healthy volunteers. Thus it used as a relevant preclinical model for pharmacological profiling of new therapeutics. [28] Methyllycaconitine- and scopolamine-induced cognitive dysfunction: differential reversal effect by cognition-enhancing drugs
Scopolamine is a non-specific muscarinic antagonist at all four muscarinic acetylcholine receptors (M1, M2, M3, and M4).,[29][30]
Biosynthesis in plants
Hyoscine is among the secondary metabolites of plants from Solanaceae (nightshade) family of plants, such as henbane (Hyoscyamus niger), jimson weed (Datura), angel's trumpets (Brugmansia), and corkwood (Duboisia).[31][10]
The biosynthesis of hyoscine begins with the decarboxylation of L-ornithine to putrescine by ornithine decarboxylase. Putrescine is methylated to N-methylputrescine by putrescine N-methyltransferase.[32]
A putrescine oxidase that specifically recognizes methylated putrescine catalyzes the deamination of this compound to 4-methylaminobutanal, which then undergoes a spontaneous ring formation to N-methyl-pyrrolium cation. In the next step, the pyrrolium cation condenses with acetoacetic acid yielding hygrine. No enzymatic activity could be demonstrated to catalyze this reaction. Hygrine further rearranges to tropinone.[32]
Subsequently, tropinone reductase I converts tropinone to tropine which condenses with phenylalanine-derived phenyllactate to littorine. A cytochrome P450 classified as Cyp80F1[33] oxidizes and rearranges littorine to hyoscyamine aldehyde. In the final step, hyoscyamine undergoes epoxidation catalyzed by 6beta-hydroxyhyoscyamine epoxidase yielding hyoscine.[32]

History
One of the earlier alkaloids isolated from plant sources, hyoscine has been in use in its purified forms (such as various salts, including hydrochloride, hydrobromide, hydroiodide and sulfate), since its isolation by the German scientist Albert Ladenburg in 1880,[34] and as various preparations from its plant-based form since antiquity and perhaps prehistoric times. Following the description of the structure and activity of hyoscine by Ladenburg, the search for synthetic analogues of and methods for total synthesis of hyoscine and/or atropine in the 1930s and 1940s resulted in the discovery of diphenhydramine, an early antihistamine and the prototype of its chemical subclass of these drugs, and pethidine, the first fully synthetic opioid analgesic, known as Dolantin and Demerol amongst many other trade names.
In 1899, a Dr. Schneiderlin recommended the use of hyoscine and morphine for surgical anaesthesia and it started to be used sporadically for that purpose.[6][35] The use of this combination in obstetric anesthesiology was first proposed by Richard von Steinbuchel in 1902 and was picked up and further developed by Carl Gauss in Freiburg, Germany starting in 1903.[36] The method came to be known as "Dämmerschlaf" ("twilight sleep") or the "Freiburg method".[35][36] It spread rather slowly, and different clinics experimented with different dosages and ingredients; in 1915 The Canadian Medical Association Journal reported that "the method [was] really still in a state of development".[35] It remained widely used in the US until the 1960s, when growing chemophobia and a desire for more natural childbirth led to its abandonment.[37]
Society and culture
Cost
The cost of extended release 1 mg/72 hr is about $49 for 4 films of scopolamine transdermal film[38]
.svg.png.webp) Hyoscine costs (US)
Hyoscine costs (US).svg.png.webp) Hyoscine prescriptions (US)
Hyoscine prescriptions (US)
Names
Hyoscine hydrobromide is the international nonproprietary name, and scopolamine hydrobromide is the United States Adopted Name. Other names include levo-duboisine, devil's breath and burundanga.[39]
Australian bush medicine
A bush medicine developed by Aboriginal peoples of the eastern states of Australia from the soft corkwood tree, or Duboisia myoporoides, was used by the Allies in World War II to stop soldiers getting seasick when they sailed across the English Channel on their way to liberate France and defeat Hitler during the Invasion of Normandy. Later, it was found that the same substance could be used in the production of scopolamine and hyoscyamine, which are used in eye surgery, and a multi-million dollar industry was built in Queensland based on this substance.[40]
Recreational and religious use
While it has been occasionally used recreationally for its hallucinogenic properties, the experiences are often unpleasant, mentally and physically. It is also physically dangerous and formally classified as a deliriant drug, so repeated use is rare.[41] In June 2008, more than 20 people were hospitalized with psychosis in Norway after ingesting counterfeit Rohypnol tablets containing hyoscine.[42] In January 2018, 9 individuals were hospitalized in Perth, Western Australia, after reportedly ingesting hyoscine.[43]
Historically, the various plants which produce hyoscine have been used psychoactively for spiritual purposes.[44][45] When entheogenic preparations of these plants were utilized, hyoscine was considered to be the main psychoactive compound and was largely responsible for the hallucinogenic effects, particularly when the preparation was made into a topical ointment (most notably flying ointment).[46] Hyoscine is reported to be the only active alkaloid within these plants that can effectively be absorbed through the skin to cause effects.[47] Different recipes for these ointments were explored in European witchcraft at least as far back as the Early Modern period and included multiple ingredients to help with the transdermal absorption of hyoscine (such as animal fat) as well as other possible ingredients to counteract its noxious and dysphoric effects.[46]
Interrogation
The effects of hyoscine were studied for use as a truth serum in interrogations in the early 20th century,[48] but because of the side effects, investigations were dropped.[49] In 2009, the Czechoslovak state security secret police were proven to have used hyoscine at least three times to obtain confessions from alleged antistate dissidents.[50]
Crime
Claims that hyoscine is commonly used in crime have been described as "exaggerated" or even implausible.[51] Powdered hyoscine, in a form referred to as 'Devil's breath', does not 'brainwash' or control people into being defrauded by their attackers; these alleged effects are most likely urban legends.[52] Nevertheless, the drug is known to produce loss of memory following exposure and sleepiness, similar to the effect of benzodiazepines or alcohol poisoning.
A travel advisory published by the United States Department of State in 2012 stated: "One common and particularly dangerous method that criminals use in order to rob a victim is through the use of drugs. The most common [in Colombia] has been hyoscine. Unofficial estimates put the number of annual hyoscine incidents in Colombia at approximately 50,000. Hyoscine can render a victim unconscious for 24 hours or more. In large doses, it can cause respiratory failure and death. It is most often administered in liquid or powder form in foods and beverages. The majority of these incidents occur in night clubs and bars, and usually men, perceived to be wealthy, are targeted by young, attractive women. It is recommended that, to avoid becoming a victim of hyoscine, a person should never accept food or beverages offered by strangers or new acquaintances, nor leave food or beverages unattended in their presence. Victims of hyoscine or other drugs should seek immediate medical attention."[53]
Beside robberies it is also allegedly involved in express kidnappings and sexual assault.[54] The Hospital Clínic in Barcelona introduced a protocol in 2008 to help medical workers identify cases, while Madrid hospitals adopted a similar working document in February 2015.[54] Hospital Clínic has found little scientific evidence to support this use and relies on the victims' stories to reach any conclusion.[54] Although poisoning by hyoscine appears quite often in the media as an aid for raping, kidnapping, killing or robbery, the effects of this drug and the way it is applied by criminals (transdermal injection, on playing cards and papers etc.) are often exaggerated,[55][56][57] especially skin exposure, as the dose that can be absorbed by the skin is too low to have any effect.[54] Hyoscine transdermal patches must be used for hours to days.[20]
The name "burundanga" derives from being an extract of the Brugmansia plant.[58]
Between 1998 and 2004, 13% of emergency room admissions for poisoning with criminal intentions in a clinic of Bogotá, Colombia have been attributed to hyoscine, and 44% to benzodiazepines.[39] Most commonly, the person has been poisoned by a robber who gave the victim a scopolamine-laced beverage, in the hope that the victim would become unconscious or unable to effectively resist the robbery.[39]
Research
Hyoscine is used as a research tool to study memory encoding. Initially, in human trials, relatively low doses of the muscarinic receptor antagonist, scopolamine, were found to induce temporary cognitive defects.[59] Since then, scopolamine has become a standard drug for experimentally inducing cognitive defects in animals.[60][61] Results in primates suggest that acetylcholine is involved in the encoding of new information into long-term memory.[62]
Hyoscine produces detrimental effects on short-term memory, memory acquisition, learning, visual recognition memory, visuospatial praxis, visuospatial memory, visuoperceptual function, verbal recall and psychomotor speed.[63][60][61] However, scopolamine does not seem to impair recognition and memory retrieval.[61] Acetylcholine projections in hippocampal neurons, which are vital in mediating long term potentiation, are inhibited by scopolamine.[61][61][64] Hyoscine also inhibits cholinergic mediated glutamate release in hippocampal neurons which assist in depolarization, potentiation of action potential, and synaptic suppression. Hyoscine's effects on acetylcholine and glutamate release in the hippocampus favors retrieval dominant cognitive functioning.[61] Hyoscine has been used to model the defects in cholinergic function for models of Alzheimer's, dementia, fragile X syndrome, and Down syndrome.[61][65][66][67]
Hyoscine has also been investigated as a rapid-onset antidepressant, with a number of small studies finding positive results.[68][69][70][71]
See also
- Hyoscine butylbromide (scopolamine butylbromide)
- Norscopolamine, a related alkaloid
References
- 1 2 3 4 5 6 7 8 "Scopolamine". The American Society of Health-System Pharmacists. Archived from the original on 7 October 2016. Retrieved 8 December 2016.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 23 October 2020. Retrieved 19 September 2020.
- ↑ Putcha L, Cintrón NM, Tsui J, Vanderploeg JM, Kramer WG (June 1989). "Pharmacokinetics and oral bioavailability of scopolamine in normal subjects". Pharmaceutical Research. 6 (6): 481–5. doi:10.1023/A:1015916423156. PMID 2762223.
- ↑ Juo, Pei-Show (2001). Concise Dictionary of Biomedicine and Molecular Biology (2nd ed.). Hoboken: CRC Press. p. 570. ISBN 9781420041309. Archived from the original on 10 September 2017.
- ↑ "Scopolamine Use During Pregnancy | Drugs.com". Drugs.com. Archived from the original on 21 December 2016. Retrieved 15 December 2016.
- 1 2 Keys, Thomas E. (1996). The history of surgical anesthesia (PDF) (Reprint ed.). Park Ridge, Ill.: Wood Library, Museum of Anesthesiology. p. 48ff. ISBN 978-0-9614932-7-1. Archived from the original (PDF) on 30 October 2020. Retrieved 1 August 2020.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 551. ISBN 9783527607495. Archived from the original on 10 September 2017. Retrieved 1 August 2020.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Osbourn, Anne E.; Lanzotti, Virginia (2009). Plant-derived Natural Products: Synthesis, Function, and Application. Springer Science & Business Media. p. 5. ISBN 9780387854984. Archived from the original on 10 September 2017.
- 1 2 The Chambers Dictionary. Allied Publishers. 1998. pp. 788, 1480. ISBN 978-81-86062-25-8.
- ↑ Cattell, Henry Ware (1910). Lippincott's new medical dictionary: a vocabulary of the terms used in medicine, and the allied sciences, with their pronunciation, etymology, and signification, including much collateral information of a descriptive and encyclopedic character. Lippincott. p. 435. Archived from the original on 10 September 2017. Retrieved 25 February 2012.
- 1 2 Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. pp. 49, 266, 822, 823. ISBN 978-0-85711-084-8.
- 1 2 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ Bitterman N, Eilender E, Melamed Y (May 1991). "Hyperbaric oxygen and scopolamine". Undersea Biomedical Research. 18 (3): 167–74. PMID 1853467. Archived from the original on 20 August 2008.
- ↑ Williams TH, Wilkinson AR, Davis FM, Frampton CM (March 1988). "Effects of transcutaneous scopolamine and depth on diver performance". Undersea Biomedical Research. 15 (2): 89–98. PMID 3363755. Archived from the original on 20 August 2008.
- ↑ "scopolamine solution - ophthalmic, Isopto". MedicineNet.com. Archived from the original on 31 March 2019. Retrieved 12 February 2019.
- ↑ Briggs (1994). Drugs in Pregnancy and Lactation. Baltimore, Maryland: Williams and Wilkins. pp. 777–778.
- ↑ "Study suggests link between long-term use of anticholinergics and dementia risk". Alzheimer's Society. 26 January 2015. Archived from the original on 12 November 2015. Retrieved 17 February 2015.
- ↑ Flicker C, Ferris SH, Serby M (1992). "Hypersensitivity to scopolamine in the elderly". Psychopharmacology. 107 (2–3): 437–41. doi:10.1007/bf02245172. PMID 1615141.
- 1 2 White PF, Tang J, Song D, Coleman JE, Wender RH, Ogunnaike B, Sloninsky A, Kapu R, Shah M, Webb T (January 2007). "Transdermal scopolamine: an alternative to ondansetron and droperidol for the prevention of postoperative and postdischarge emetic symptoms". Anesthesia and Analgesia. 104 (1): 92–6. doi:10.1213/01.ane.0000250364.91567.72. PMID 17179250. Archived from the original on 28 August 2021. Retrieved 1 August 2020.
- ↑ "Transderm Scop patch prescribing information". Archived from the original on 4 February 2009.
- ↑ "NASA Signs Agreement to Develop Nasal Spray for Motion Sickness".
{{cite web}}: CS1 maint: url-status (link) - ↑ "TRANSDERM SCOP (scopalamine) patch, extended release [Baxter Healthcare Corporation]". DailyMed. Baxter Healthcare Corporation. April 2013. Archived from the original on 23 October 2013. Retrieved 22 October 2013.
- ↑ "DBL™ HYOSCINE INJECTION BP". TGA eBusiness Services. Hospira Australia Pty Ltd. 30 January 2012. Archived from the original on 30 March 2017. Retrieved 22 October 2013.
- 1 2 "Buscopan Tablets - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Boehringer Ingelheim Limited. 11 September 2013. Archived from the original on 23 October 2013. Retrieved 22 October 2013.
- 1 2 3 "Kwells 300 microgram tablets - Summary of Product Characteristics". electronic Medicines Compendium. Bayer plc. 7 January 2008. Archived from the original on 23 October 2013. Retrieved 22 October 2013.
- ↑ Paul G. Barash; et al., eds. (2009). Clinical anesthesia (6 ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 346. ISBN 978-0-7817-8763-5.
- ↑ Pharmacol Res Perspect. 2014 Aug;2(4):e00048. doi: 10.1002/prp2.48. Epub 2014 Jun 9
- ↑ "Google Scholar". scholar.google.com. Archived from the original on 30 October 2020. Retrieved 16 December 2017.
- ↑ "PDSP Ki Database". Archived from the original on 23 July 2020. Retrieved 1 August 2020.
- ↑ Muranaka T, Ohkawa H, Yamada Y (1993). "Continuous Production of Scopolamine by a Culture of Duboisia leichhardtii Hairy Root Clone in a Bioreactor System". Applied Microbiology and Biotechnology. 40 (2–3): 219–223. doi:10.1007/BF00170370.
- 1 2 3 Ziegler J, Facchini PJ (2008). "Alkaloid biosynthesis: metabolism and trafficking". Annual Review of Plant Biology. 59 (1): 735–69. doi:10.1146/annurev.arplant.59.032607.092730. PMID 18251710.
- ↑ Li R, Reed DW, Liu E, Nowak J, Pelcher LE, Page JE, Covello PS (May 2006). "Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement". Chemistry & Biology. 13 (5): 513–20. doi:10.1016/j.chembiol.2006.03.005. PMID 16720272.
- ↑ Ladenburg, Albert (1881). "Die natürlich vorkommenden mydriatisch wirkenden Alkaloïde" [The naturally occurring alkaloids that act mydriatically [i.e., to dilate the pupils]]. Annalen der Chemie (in German). 206 (3): 274–307. doi:10.1002/jlac.18812060303. Archived from the original on 29 October 2020. Retrieved 1 August 2020.
{{cite journal}}: CS1 maint: unrecognized language (link); see pp. 299–307. - 1 2 3 "Twilight Sleep: the Dammerschlaf of the Germans". Canadian Medical Association Journal. 5 (9): 805–8. September 1915. PMC 1584452. PMID 20310688.
- 1 2 "TWILIGHT SLEEP; Is Subject of a New Investigation". The New York Times. 31 January 1915. Archived from the original on 4 March 2016. Retrieved 1 August 2020.
- ↑ Finkbeiner, Ann (31 October 1999). "Labor Dispute. Book review: What a Blessing She Had Chloroform: The Medical and Social Response to the Pain of Childbirth from 1800 to the Present". New York Times. Archived from the original on 30 October 2020. Retrieved 1 August 2020.
- ↑ "Scopolamine Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 23 January 2021. Retrieved 17 March 2021.
- 1 2 3 Uribe M, Moreno CL, Zamora A, Acosta P (September 2005). "Perfil epidemiológico de la intoxicación con burundanga en la clínica Uribe Cualla S. A. de Bogotá, D. C" (PDF). Acta Neurológica Colombiana (in Spanish). 21 (3): 197–201. Archived (PDF) from the original on 7 October 2016.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ "Visitors to Art of Healing exhibition told how Australian Indigenous bush medicine was given to every allied soldier landing at Normandy on D-Day". King's College London. 7 June 2019. Archived from the original on 2 June 2020. Retrieved 2 June 2020.
- ↑ Freye E (2010). "Toxicity of Datura Stramonium". Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs. Netherlands: Springer. pp. 217–218. doi:10.1007/978-90-481-2448-0_34. ISBN 978-90-481-2447-3.
- ↑ "Bilsykemedisin i falske rohypnol-tabletter". Aftenposten.no. Archived from the original on 27 June 2008.
- ↑ "Perth backpacker overdose linked to common anti-nausea drug". ABC News. 4 January 2018. Archived from the original on 7 November 2020. Retrieved 4 January 2018.
- ↑ Raetsch, Ch. (2005). The encyclopedia of psychoactive plants: ethnopharmacology and its applications. US: Park Street Press. pp. 277–282.
- ↑ Harner, Michael (1980). The Way of the Shaman. New York: Harper & Row.
- 1 2 Hansen, Harold A. The Witch's Garden pub. Unity Press 1978 ISBN 978-0913300473
- ↑ Sollmann, Torald, A Manual of Pharmacology and Its Applications to Therapeutics and Toxicology. 8th edition. Pub. W.B. Saunders, Philadelphia and London 1957.
- ↑ House RE (September 1922). "The Use of Scopolamine in Criminology". Texas State Journal of Medicine. 18: 256–263.
Reprinted in: House RE (July–August 1931). "The Use of Scopolamine in Criminology". American Journal of Police Science. 2 (4): 328–336. doi:10.2307/1147361. JSTOR 1147361. - ↑ Bimmerle, George (22 September 1993). "'Truth' Drugs in Interrogation". Central Intelligence Agency. Archived from the original on 27 September 2012. Retrieved 14 June 2012.
- ↑ Gazdík J, Navara L (8 August 2009). "Svědek: Grebeníček vězně nejen mlátil, ale dával jim i drogy" [A witness: Grebeníček not only beat prisoners, he also administered drugs to them] (in Czech). iDnes. Archived from the original on 11 August 2009. Retrieved 10 August 2009.
{{cite news}}: CS1 maint: unrecognized language (link) - ↑ Saner, Emine (2 September 2015). "'Devil's breath' aka scopolamine: can it really zombify you?". The Guardian. Archived from the original on 4 January 2019. Retrieved 4 January 2019.
- ↑ Anderson, L. "Devil's Breath: Urban Legend or the World's Most Scary Drug?". Drugs.com. Archived from the original on 23 June 2019. Retrieved 9 July 2019.
- ↑ "Colombia 2012 Crime and Safety Report: Cartagena". Overseas Security Advisory Council, United States Department of State. 4 March 2012. Archived from the original on 15 March 2013. Retrieved 6 August 2015.
- 1 2 3 4 Domínguez, Iñigo (25 July 2016). "Burundanga: the stealth drug that cancels the victim's willpower". Crime. El País, Madrid. Archived from the original on 20 August 2016. Retrieved 12 August 2016.
- ↑ ""Burundanga Business Card Drug Warning". Hoax-Slayer.com". 12 October 2008. Archived from the original on 7 March 2009.
- ↑ "Beware the Burundanga Man!". About.com Entertainment. Archived from the original on 10 January 2017. Retrieved 19 December 2016.
- ↑ Mikkelson, David. "Burundanga/Scopolamine Warning". snopes. Retrieved 19 December 2016.
- ↑ Vaughan Bell (3 March 2011). "Mind controller: What is the 'burundanga' drug?". Wired UK. Archived from the original on 11 August 2017.
{{cite magazine}}: Unknown parameter|publicationdate=ignored (help) - ↑ Drachman DA, Leavitt J (February 1974). "Human memory and the cholinergic system. A relationship to aging?". Archives of Neurology. 30 (2): 113–21. doi:10.1001/archneur.1974.00490320001001. PMID 4359364.
- 1 2 Hasselmo ME, Wyble BP (December 1997). "Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function". Behavioural Brain Research. 89 (1–2): 1–34. doi:10.1016/s0166-4328(97)00048-x. PMID 9475612.
- 1 2 3 4 5 6 7 More SV, Kumar H, Cho DY, Yun YS, Choi DK (September 2016). "Toxin-Induced Experimental Models of Learning and Memory Impairment". International Journal of Molecular Sciences. 17 (9): 1447. doi:10.3390/ijms17091447. PMC 5037726. PMID 27598124.
- ↑ Ridley RM, Bowes PM, Baker HF, Crow TJ (1984). "An involvement of acetylcholine in object discrimination learning and memory in the marmoset". Neuropsychologia. 22 (3): 253–63. doi:10.1016/0028-3932(84)90073-3. PMID 6431311.
- ↑ Flicker C, Serby M, Ferris SH (February 1990). "Scopolamine effects on memory, language, visuospatial praxis and psychomotor speed". Psychopharmacology. 100 (2): 243–50. doi:10.1007/bf02244414. PMID 2305013.
- ↑ Lisboa SF, Vila-Verde C, Rosa J, Uliana DL, Stern CA, Bertoglio LJ, Resstel LB, Guimaraes FS (January 2019). "Tempering aversive/traumatic memories with cannabinoids: a review of evidence from animal and human studies". Psychopharmacology. 236 (1): 201–226. doi:10.1007/s00213-018-5127-x. PMID 30604182.
- ↑ Qin M, Zeidler Z, Moulton K, Krych L, Xia Z, Smith CB (September 2015). "Endocannabinoid-mediated improvement on a test of aversive memory in a mouse model of fragile X syndrome". Behavioural Brain Research. 291: 164–171. doi:10.1016/j.bbr.2015.05.003. PMC 5003021. PMID 25979787.
- ↑ Lott IT (2012). "Neurological phenotypes for Down syndrome across the life span". Progress in Brain Research. 197: 101–21. doi:10.1016/b978-0-444-54299-1.00006-6. ISBN 9780444542991. PMC 3417824. PMID 22541290.
- ↑ Lagalwar S, Bordayo EZ, Hoffmann KL, Fawcett JR, Frey WH (1999). "Anandamides inhibit binding to the muscarinic acetylcholine receptor". Journal of Molecular Neuroscience. 13 (1–2): 55–61. doi:10.1385/jmn:13:1-2:55. PMID 10691292.
- ↑ Drevets WC, Zarate CA, Furey ML (June 2013). "Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review". Biological Psychiatry. 73 (12): 1156–63. doi:10.1016/j.biopsych.2012.09.031. PMC 4131859. PMID 23200525.
- ↑ Hasselmann H (2014). "Scopolamine and depression: a role for muscarinic antagonism?". CNS & Neurological Disorders Drug Targets. 13 (4): 673–83. doi:10.2174/1871527313666140618105710. PMID 24938776.
- ↑ Jaffe RJ, Novakovic V, Peselow ED (2013). "Scopolamine as an antidepressant: a systematic review". Clinical Neuropharmacology. 36 (1): 24–6. doi:10.1097/wnf.0b013e318278b703. PMID 23334071.
- ↑ Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, Duman RS (July 2016). "GABA interneurons mediate the rapid antidepressant-like effects of scopolamine". The Journal of Clinical Investigation. 126 (7): 2482–94. doi:10.1172/JCI85033. PMC 4922686. PMID 27270172.
External links
| Identifiers: |
|---|
- "Hyoscine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 30 September 2020. Retrieved 1 August 2020.