Netupitant
 | |
| Clinical data | |
|---|---|
| License data | |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | >60% (estimated) |
| Protein binding | >99% |
| Metabolism | mainly CYP3A4; also CYP2D6 and CYP2C9 |
| Elimination half-life | 88 hours |
| Excretion | 71% (faeces) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C30H32F6N4O |
| Molar mass | 578.603 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Netupitant is an antiemetic drug. In the United States, the combination drug netupitant/palonosetron (trade name Akynzeo) is approved by the Food and Drug Administration for prevention of acute and delayed chemotherapy-induced nausea and vomiting, including highly emetogenic chemotherapy such as with cisplatin.[1][2] In Europe, it is approved by the European Medicines Agency (EMA) for the same indication.[3][4]
Adverse effects
Side effects of the combination netupitant/palonosetron are similar to palonosetron alone, so that no common side effects can be attributed to netupitant.[3][1]
headache, weakness, indigestion, fatigue, constipation, and skin redness
Interactions
Netupitant blood plasma levels are expected to increase when combined with inhibitors of the liver enzyme CYP3A4 and lowered when combined with inductors of this enzyme.[3]
Being a CYP3A4 inhibitor itself, netupitant could also increase plasma levels of pharmaceuticals that are metabolized by CYP3A4. This effect has been observed with dexamethasone, the anti-cancer drugs docetaxel and etoposide, and to a minor (not clinically significant) extent with levonorgestrel, erythromycin and midazolam.[3]
Pharmacology
Mechanism of action
Netupitant is a selective NK1 receptor antagonist.[5]
Netupitant is a selective neurokinin 1 (NK1) receptor antagonist with potential antiemetic activity. Netupitant competitively binds to and blocks the activity of the human substance P/NK1 receptors in the central nervous system (CNS), thereby inhibiting NK1-receptor binding of the endogenous tachykinin neuropeptide substance P (SP), which may result in the prevention of chemotherapy-induced nausea and vomiting (CINV). SP is found in neurons of vagal afferent fibers innervating the brain-stem nucleus tractus solitarii and the area postrema, which contains the chemoreceptor trigger zone (CTZ), and may be elevated in response to chemotherapy. The NK-receptor is a G-protein receptor coupled to the inositol phosphate signal-transduction pathway and is found in both the nucleus tractus solitarii and the area postrema.[6]
Pharmacokinetics
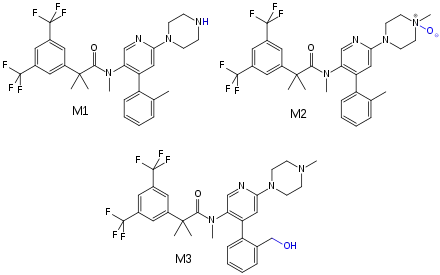
Bioavailability is estimated to be over 60% for orally taken netupitant. Highest blood plasma concentrations are reached five hours after application. Availability is moderately (10–20%) increased when taken after a fatty meal. Netupitant and its main metabolites (called M1 and M3) are bound to plasma proteins to more than 99%, and M2 protein binding is 97%.[3]
The substance is mainly metabolized by CYP3A4, and to a lesser extent by CYP2D6 and CYP2C9. The main metabolites are desmethyl-netupitant (M1), netupitant N-oxide (M2), and hydroxy-netupitant (M3); all three are pharmacologically active.[3][7]
Netupitant and its metabolites are mainly excreted via the faeces.[3] Biological half-life is 88 hours, significantly longer than that of the first NK1 receptor antagonist, aprepitant, which has a half-life of 9 to 13 hours.[8]
References
- 1 2 "Akynzeo- netupitant and palonosetron capsule Akynzeo- fosnetupitant and palonosetron injection". DailyMed. 30 April 2018. Retrieved 19 March 2020.
- ↑ "FDA approves Akynzeo for nausea and vomiting associated with cancer chemotherapy". Food and Drug Administration. October 10, 2014. Archived from the original on February 1, 2017. Retrieved December 16, 2019.
- 1 2 3 4 5 6 7 "Akynzeo: Summary of Product Characteristics" (PDF). European Medicines Agency. Retrieved 12 July 2016.
- ↑ "Akynzeo EPAR". European Medicines Agency (EMA). 19 March 2020. Retrieved 19 March 2020.
- ↑ Rizzi A, Campi B, Camarda V, Molinari S, Cantoreggi S, Regoli D, Pietra C, Calo G (2012). "In vitro and in vivo pharmacological characterization of the novel NK(1) receptor selective antagonist netupitant". Peptides. 37 (1): 86–97. doi:10.1016/j.peptides.2012.06.010. PMID 22732666. S2CID 7982557.
- ↑ "Netupitant".
- 1 2 Spinelli, T; Calcagnile, S; Giuliano, C; Rossi, G; Lanzarotti, C; Mair, S; Stevens, L; Nisbet, I (2013). "Netupitant PET imaging and ADME studies in humans". The Journal of Clinical Pharmacology. 54 (1): 97–108. doi:10.1002/jcph.198. PMC 4282341. PMID 24122871.
- ↑ Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
External links
- "Netupitant". Drug Information Portal. U.S. National Library of Medicine.
- "Netupitant mixture with palonosetron". Drug Information Portal. U.S. National Library of Medicine.