Ibodutant
 | |
| Clinical data | |
|---|---|
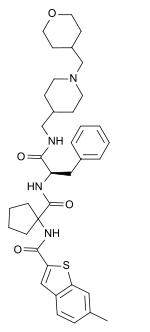
| Other names | 6-methyl-N-[1-[[(2R)-1-[[1-(oxan-4-ylmethyl)piperidin-4- yl]methylamino]-1-oxo-3-phenylpropan-2-yl]carbamoyl]cyclopentyl]-1-benzothiophene-2-carboxamide |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C37H48N4O4S |
| Molar mass | 644.88 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Ibodutant was a candidate drug for irritable bowel syndrome diarrhea, developed by The Menarini Group. As of March 2015, it underwent a multicentre double blind efficacy clinical study. Ibodutant selectively blocks the tachykinin receptor NK2, with blockade practically complete in nanomolar concentrations. A phase 2 trial in Europe (the IRIS-2 trial) completed in May 2012 with positive results. A 52-week phase 3 study was terminated as of 2015 because of low response and negative results of study NAK-06.[1]
See also
References
- ↑ "Clinical Trials Database". Menarini Group. Retrieved 11 March 2015.
Further reading
- Spreitzer H (May 26, 2008). "Neue Wirkstoffe - Ibodutant". Österreichische Apothekerzeitung (in German) (11/2008): 541.
- Giuliani S, Altamura M, Maggi CA. "Ibodutant. Tachykinin NK2 receptor antagonist, Treatment of irritable bowel syndrome". Drugs of the Future. 33 (2): 111–115. doi:10.1358/dof.2008.033.02.1181381.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.