Ispronicline
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
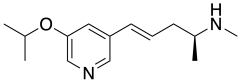
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C14H22N2O |
| Molar mass | 234.336 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Ispronicline (TC-1734, AZD-3480) is an experimental drug which acts as a partial agonist at neural nicotinic acetylcholine receptors. It progressed to phase II clinical trials for the treatment of dementia and Alzheimer's disease, but is no longer under development.[1]
It has also progressed to phase II as a potential treatment for ADHD. With dosages of 50mg day showing a significant improvement in ADHD symptoms[2][3]
Ispronicline is subtype-selective, binding primarily to the α4β2 subtype. It has antidepressant, nootropic and neuroprotective effects.
Early stage clinical trials showed that ispronicline was well tolerated, with the main side effects being dizziness and headache.[4][5][6][7][8] However, mid stage clinical trials failed to show sufficient efficacy to continue development as a pharmaceutical drug.[1]
See also
References
- 1 2 Targacept Drops Development of Alzheimer's Drug
- ↑ Potter, Alexandra S.; Dunbar, Geoffrey; Mazzulla, Emily; Hosford, David; Newhouse, Paul A. (2014-02-01). "AZD3480, a novel nicotinic receptor agonist, for the treatment of attention-deficit/hyperactivity disorder in adults". Biological Psychiatry. 75 (3): 207–214. doi:10.1016/j.biopsych.2013.06.002. ISSN 1873-2402. PMID 23856296. S2CID 44717647.
- ↑ AstraZeneca (2009-10-29). "An Exploratory Trial of AZD3480 (TC-1734) for the Treatment of Adult Attention-Deficit/Hyperactivity Disorder (ADHD)". Targacept Inc.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Gatto GJ, Bohme GA, Caldwell WS, Letchworth SR, Traina VM, Obinu MC, et al. (2004). "TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects". CNS Drug Reviews. 10 (2): 147–66. doi:10.1111/j.1527-3458.2004.tb00010.x. PMC 6741718. PMID 15179444.
- ↑ Dunbar G, Demazières A, Monreal A, Cisterni C, Metzger D, Kuchibhatla R, Luthringer R (July 2006). "Pharmacokinetics and safety profile of ispronicline (TC-1734), a new brain nicotinic receptor partial agonist, in young healthy male volunteers". Journal of Clinical Pharmacology. 46 (7): 715–26. doi:10.1177/0091270006288730. PMID 16809797. S2CID 22499622.
- ↑ Lippiello P, Letchworth SR, Gatto GJ, Traina VM, Bencherif M (2006). "Ispronicline: a novel alpha4beta2 nicotinic acetylcholine receptor-selective agonist with cognition-enhancing and neuroprotective properties". Journal of Molecular Neuroscience. 30 (1–2): 19–20. doi:10.1385/JMN:30:1:19. PMID 17192610.
- ↑ Dunbar G, Boeijinga PH, Demazières A, Cisterni C, Kuchibhatla R, Wesnes K, Luthringer R (May 2007). "Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers". Psychopharmacology. 191 (4): 919–29. doi:10.1007/s00213-006-0675-x. PMID 17225162. S2CID 10920515.
- ↑ Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J (March 2007). "Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI)". Journal of Psychopharmacology. 21 (2): 171–8. doi:10.1177/0269881107066855. PMID 17329297. S2CID 10056476.