Azacyclonol
 | |
| Clinical data | |
|---|---|
| Other names | MER-17; MDL-4829; Diphenylmethanolpiperidine |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.720 |
| Chemical and physical data | |
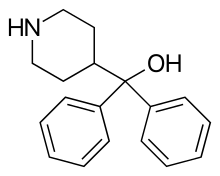
| Formula | C18H21NO |
| Molar mass | 267.372 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Azacyclonol (trade names Ataractan, Calmeran, Frenoton, Frenquel, Psychosan), also known as γ-pipradrol, is a drug which is an ataractive; an agent which diminishes hallucinations in psychotic individuals.[1][2] It has also been called a tranquilizer and antipsychotic, though these definitions are not accurate as it does not actually possess such properties. Despite being a positional isomer of pipradrol, it is not a psychostimulant, and instead has mild depressant effects.[1][3]
The drug was introduced in Europe in the mid-1950s for the treatment of schizophrenia likely because it was found to attenuate the subjective psychedelic effects of LSD and mescaline in humans.[1][4] However, due to poor and mixed clinical effectiveness,[4] it never gained widespread acceptance and was eventually discontinued.
Azacyclonol is also known as diphenylmethanolpiperidine and is the parent structure of the antihistamines fexofenadine and terfenadine. Terfenadine produces azacyclonol as a major active metabolite.[5]
It is made by the organometallic addition of 4-bromopyridine to benzophenone, followed by catalytic hydrogenation of the pyridine heteroaromatic ring system to the corresponding piperidine.[6]
References
- 1 2 3 BRAUN DL, BROWN BB, FELDMAN RG (October 1956). "The pharmacologic activity of alpha-(4-piperidyl)-benzhydrol hydrochloride (azacyclonol hydrochloride); an ataractive agent". The Journal of Pharmacology and Experimental Therapeutics. 118 (2): 153–61. PMID 13368052.
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ↑ FARRANT J (June 1963). "Interactions between cocaine, tyramine and noradrenaline at the noradrenaline store". British Journal of Pharmacology and Chemotherapy. 20 (3): 540–9. doi:10.1111/j.1476-5381.1963.tb01491.x. PMC 1703814. PMID 13944436.
- 1 2 FORSTER W, HENDERSON AL (January 1957). "A clinical study of Frenquel (alpha (4-piperidyl) benzhydrol hydrochloride) in chronic schizophrenia". Canadian Medical Association Journal. 76 (2): 97–101. PMC 1823487. PMID 13383414.
- ↑ Martens J (April 1996). "Determination of the terfenadine metabolite azacyclonol in human serum using gas chromatography-mass spectrometry". Journal of Chromatography B. 678 (2): 349–53. doi:10.1016/0378-4347(95)00561-7. PMID 8738042.
- ↑ Campen Jr Marcus G Van, Pogge Raymond C, Schumann Edward L; Wm S Merrell Co; U.S. Patent 2,804,422 (1957).