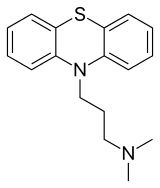
Promazine
 | |
 | |
| Names | |
|---|---|
| Pronunciation | proe-ma-zeen[1] |
| Trade names | Sparine, Prozine, Primazine, others[1] |
IUPAC name
| |
| Clinical data | |
| Drug class | Typical antipsychotic |
| Main uses |
|
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600010 |
| Pharmacokinetics | |
| Protein binding | 94% |
| Elimination half-life | 20-40 hr |
| Chemical and physical data | |
| Formula | C17H20N2S |
| Molar mass | 284.42 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Promazine, sound under the brand name Sparine among others,[1] is used as a short-term add-on treatment for psychomotor agitation or for agitation and restlessness in the elderly.[2][3] Its approved uses in people is limited, but is used as a tranquilizer in veterinary medicine.[2] It has weak antipsychotic effects but is generally not used to treat psychoses.[2] It can be given as a liquid or tablet by mouth, or by injection.[4]
It acts similar to chlorpromazine and causes sedation.[2] It has predominantly anticholinergic side effects, though extrapyramidal side effects are not uncommon. It belongs to the typical antipsychotic and phenothiazine class of drugs.[5]
Promazine was approved for medical use in the United States in 1957, although it is no longer commercially available there.[1][6][7]
Medical uses
Promazine is a short-term add-on treatment for psychomotor agitation.[3] It can be used to manage nausea and vomiting in people using cancer treatments.[4] It can be given as a liquid or tablet by mouth, or by injection.[4]
The adult dose by mouth is 100–200 mg 4 times a day.[3]
In the elderly, it can be given for restlessness and agitation at a lower dose of 25–50 mg 4 times a day by mouth.[3]
Side effects
Common side effects include agitation, absent menstruation, arrhythmias, constipation, drowsiness and dizziness, dry mouth, problems with erection, tiredness, milky nipple discharge, large breasts, high sugars, difficulty sleeping, low blood pressure, prolonged QT, fits, shaking, vomiting and weight gain, among others.[8]
Overdose
In overdose, it may cause blood pressure to drop, lowering of body temperature, increased heart rate, and an irregular heart beat.[8]
Sudden death may occur, although rare.[8]
Other animals
Promazine, given as promazine hydrochloride, is one of the primary tranquilizers used by veterinarians as a pre-anaesthesia injection in horses.[9][10] It does not provide analgesia and is not a very strong sedative, hence it is used combined with opioids or α2 adrenoreceptor agonists, such as atropine, or both.[10][11] It can be used alone when performing a non-painful procedure such as the fitting a horseshoe.[11] Low blood pressure, fast heart rate and paralysis of the penis are side effects.[9] It is also an antiemetic, antispasmodic and hypothermic agent.[10] Additionally it is used to lower blood pressure in animals suffering from laminitis and kidney failure.[10] It is available in the US for veterinary use under the names Promazine and Tranquazine.
References
- 1 2 3 4 "Promazine (Primazine, Prozine) | Davis's Drug Guide". www.drugguide.com. Archived from the original on 25 January 2021. Retrieved 5 August 2021.
- 1 2 3 4 5 6 Davis, Claire (2007). Enna, S.J.; Bylund, David B. (eds.). Promazine. Elsevier. pp. 1–6. ISBN 978-0-08-055232-3. Archived from the original on 2021-08-29. Retrieved 2021-08-06.
- 1 2 3 4 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 424. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 3 Ritter, James M.; Flower, Rod; Henderson, Graeme; Loke, Yoon Kong; Rang, Humphrey P. (2020). "31. The gastrointestinal tract". Rang & Dale's Pharmacology. Elsevier. p. 403. ISBN 978-0-7020-7448-6. Archived from the original on 2021-08-28. Retrieved 2021-08-08.
- ↑ Pagliaro LA, Pagliaro AM (1999). "PPDR: Promazine". Psychologist's Neuropsychotropic Desk Reference. Philadelphia: Brunner/Mazel. p. 535. ISBN 978-1-138-00968-4. Archived from the original on 2021-08-29. Retrieved 2021-08-04.
- ↑ Approved Drug Products With Therapeutic Equivalance Evaluations - FDA Orange Book 26th Edition (2006) (in русский) (26 ed.). DrugPatentWatch.com. 2006. p. 1667. ISBN 978-1-934899-76-2. Archived from the original on 2021-08-29. Retrieved 2021-08-05.
- ↑ "Drugs@FDA: FDA-Approved Drugs". New Drug Application (NDA): 010942 Sparine. U.S. Food and Drug Administration. Archived from the original on 2021-03-24. Retrieved 2021-08-04.
- 1 2 3 "BNF". NICE. Archived from the original on 29 August 2021. Retrieved 4 August 2021.
- 1 2 Hendrickson, Dean A. (2007). "2. Anaesthesia and fluid therapy". Techniques in Large Animal Surgery (3rd ed.). Blackwell Publishing. p. 16. ISBN 978-0-7817-8255-5. Archived from the original on 2021-08-29. Retrieved 2021-08-04.
- 1 2 3 4 "Promazine | Equimed - Horse Health Matters". EquiMed. 20 January 2014. Archived from the original on 4 August 2021. Retrieved 4 August 2021.
- 1 2 Ringer, Simone K.; Mama, Khursheed R. (2019). "23. Chemical restraint for standing procedures". In Auer, Jorg A.; Stick, John A. (eds.). Equine Surgery (5th ed.). St Louis, Missouri: Elsevier. pp. 351–352. ISBN 978-0-323-48420-6. Archived from the original on 2021-08-29. Retrieved 2021-08-04.
External links
| Identifiers: |
|---|
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|