Epinastine
 | |
 | |
| Names | |
|---|---|
| Trade names | Alesion, Elestat, Purivist, Relestat |
| Other names | Epinastine hydrochloride |
IUPAC name
| |
| Clinical data | |
| Drug class | Antihistamine[1] |
| Main uses | Allergic conjunctivitis[1] |
| Side effects | Eye irritation[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Eye drops |
| Onset of action | Within 5 min[1] |
| Duration of action | 8 hrs[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604011 |
| Pharmacokinetics | |
| Protein binding | 64% |
| Elimination half-life | 12 hours |
| Chemical and physical data | |
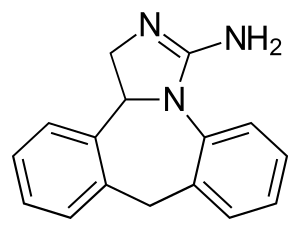
| Formula | C16H15N3 |
| Molar mass | 249.317 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Epinastine, sold under the brand name Elestat among others, is a medication used to treat allergic conjunctivitis.[1] It is used as an eye drop.[1] Effects begin within 5 minutes and last for up to 8 hours.[1] It may be used for up to 8 weeks.[2]
Common side effects include eye irritation.[2] Other side effects may include dry eye and change in taste.[2] It is an antihistamine and mast cell stabilizer.[1] It does not cross the blood-brain-barrier.[1]
Epinastine was patented in 1980 and came into medical use in 1994.[3] In the United Kingdom 5 ml cost the NHS about £10 as of 2021.[2] This amount in the United States costs about 31 USD.[4]
Medical uses
Dosage
References
- 1 2 3 4 5 6 7 8 9 10 "Epinastine Monograph for Professionals". Drugs.com. Archived from the original on 1 March 2021. Retrieved 15 December 2021.
- 1 2 3 4 5 6 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1205. ISBN 978-0857114105.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 549. ISBN 9783527607495. Archived from the original on 2020-12-27. Retrieved 2021-03-08.
- ↑ "Epinastine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 8 May 2016. Retrieved 15 December 2021.
External links
| Identifiers: |
|---|
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.