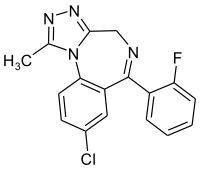
Flualprazolam
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H12ClFN4 |
| Molar mass | 326.76 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Flualprazolam is a tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It was first synthesised in 1976,[2] but was never marketed. It has subsequently been sold as a designer drug,[3][4][5] first being definitively identified as such in Sweden in 2018.[6][7] It can be described as the 2'-fluoro derivative of alprazolam, or the fluoro instead of chloro analogue of triazolam, and has similar sedative and anxiolytic effects.[8][9][10][11][12]
Legal status
Flualprazolam is banned in Sweden, also is illegal in the UK.[13] In December 2019, the World Health Organization recommended flualprazolam for international scheduling as a Schedule IV medication under the Convention on Psychotropic Substances.[14]
See also
References
- ↑ https://www.oregon.gov/pharmacy/Documents/Permanently_Adopted_TrackedChanges_Div_041_Div_080_BP_8-2020.pdf
- ↑ US 3987052, Hester JB, et al., "6-Phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines."
- ↑ Wagmann L, Manier SK, Bambauer TP, Felske C, Eckstein N, Flockerzi V, Meyer MR (February 2020). "Toxicokinetics and analytical toxicology of flualprazolam: metabolic fate, isozyme mapping, human plasma concentration, and main urinary excretion products". Journal of Analytical Toxicology. 44 (6): 549–558. doi:10.1093/jat/bkaa019. PMID 32104896.
- ↑ Papsun, Donna M.; Krotulski, Alex J.; Homan, Joe; Temporal, Keith D. H.; Logan, Barry K. (June 2020). "Flualprazolam Blood Concentrations in 197 Forensic Investigation Cases". Journal of Analytical Toxicology. doi:10.1093/jat/bkaa070. PMID 32542312.
- ↑ Rice, Kathleen; Hikin, Laura; Lawson, Alexander; Smith, Paul R.; Morley, Stephen (August 2020). "Quantification of Flualprazolam in Blood by LC–MS-MS: A Case Series of Nine Deaths". Journal of Analytical Toxicology. doi:10.1093/jat/bkaa098. PMID 32780842.
- ↑ Svenska Narkotika Polisföreningens Tidskrift, June 1 2018
- ↑ Kriikku P, Rasanen I, Ojanperä I, Thelander G, Kronstrand R, Vikingsson S (February 2020). "Femoral blood concentrations of flualprazolam in 33 postmortem cases". Forensic Science International. 307: 110101. doi:10.1016/j.forsciint.2019.110101. hdl:10138/323513. PMID 31865266.
- ↑ Waters L, Manchester KR, Maskell PD, Haegeman C, Haider S (May 2018). "The use of a quantitative structure-activity relationship (QSAR) model to predict GABA-A receptor binding of newly emerging benzodiazepines" (PDF). Science & Justice. 58 (3): 219–225. doi:10.1016/j.scijus.2017.12.004. PMID 29685303.
- ↑ Zawilska JB, Wojcieszak J (July 2019). "An expanding world of new psychoactive substances-designer benzodiazepines". Neurotoxicology. 73: 8–16. doi:10.1016/j.neuro.2019.02.015. PMID 30802466.
- ↑ Moosmann B, Auwärter V (October 2018). "Designer Benzodiazepines: Another Class of New Psychoactive Substances". Handbook of Experimental Pharmacology. Springer International Publishing. 252: 383–410. doi:10.1007/164_2018_154. ISBN 978-3-030-10561-7. PMID 30367253.
- ↑ Chetraru E, Ameline A, Gheddar L, Raul JS, Kintz P (February 2018). "Les "designer benzodiazepines" : qu'en sait-on aujourd'hui ?". Toxicologie Analytique et Clinique. 30 (1): 5–18. doi:10.1016/j.toxac.2017.12.001. ISSN 2352-0078.
- ↑ Mei V, Concheiro M, Pardi J, Cooper G (October 2019). "Validation of an LC-MS/MS Method for the Quantification of 13 Designer Benzodiazepines in Blood". Journal of Analytical Toxicology. 43 (9): 688–695. doi:10.1093/jat/bkz063. PMID 31436813.
- ↑ Riksdagsförvaltningen. "Förordning (1992:1554) om kontroll av narkotika". riksdagen.se (in Swedish).
- ↑ "News: December 2019 – WHO: World Health Organization recommends 12 NPS for scheduling". www.unodc.org.