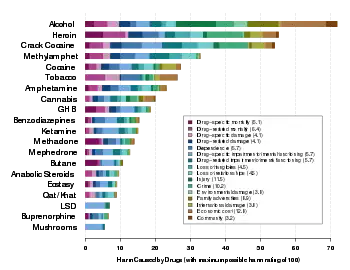
Physical dependence
Physical dependence is a physical condition caused by chronic use of a tolerance-forming drug, in which abrupt or gradual drug withdrawal causes unpleasant physical symptoms.[1][2] Physical dependence can develop from low-dose therapeutic use of certain medications such as benzodiazepines, opioids, antiepileptics and antidepressants, as well as the recreational misuse of drugs such as alcohol, opioids and benzodiazepines. The higher the dose used, the greater the duration of use, and the earlier age use began are predictive of worsened physical dependence and thus more severe withdrawal syndromes. Acute withdrawal syndromes can last days, weeks or months. Protracted withdrawal syndrome, also known as post-acute-withdrawal syndrome or "PAWS", is a low-grade continuation of some of the symptoms of acute withdrawal, typically in a remitting-relapsing pattern, often resulting in relapse and prolonged disability of a degree to preclude the possibility of lawful employment. Protracted withdrawal syndrome can last for months, years, or depending on individual factors, indefinitely. Protracted withdrawal syndrome is noted to be most often caused by benzodiazepines.[3] To dispel the popular misassociation with addiction, physical dependence to medications is sometimes compared to dependence on insulin by persons with diabetes.[4]
Symptoms and signs
| Addiction and dependence glossary[5][6][7][8] | |
|---|---|
| |
Physical dependence can manifest itself in the appearance of both physical and psychological symptoms which are caused by physiological adaptions in the central nervous system and the brain due to chronic exposure to a substance. Symptoms which may be experienced during withdrawal or reduction in dosage include increased heart rate and/or blood pressure, sweating, and tremors.[9] More serious withdrawal symptoms such as confusion, seizures, and visual hallucinations indicate a serious emergency and the need for immediate medical care. Sedative hypnotic drugs such as alcohol, benzodiazepines, and barbiturates are the only commonly available substances that can be fatal in withdrawal due to their propensity to induce withdrawal convulsions. Abrupt withdrawal from other drugs, such as opioids can cause an extremely painful withdrawal that is very rarely fatal in patients of general good health and with medical treatment, but is more often fatal in patients with weakened cardiovascular systems; toxicity is generally caused by the often-extreme increases in heart rate and blood pressure (which can be treated with clonidine), or due to arrhythmia due to electrolyte imbalance caused by the inability to eat, and constant diarrhea and vomiting (which can be treated with loperamide and ondansetron respectively) associated with acute opioid withdrawal, especially in longer-acting substances where the diarrhea and emesis can continue unabated for weeks, although life-threatening complications are extremely rare, and nearly non-existent with proper medical management.
Drugs that cause physical dependence
- All µ-opioids with any (even slight) agonist effect, such as (partial list) morphine, heroin, codeine, oxycodone, buprenorphine, nalbuphine, methadone, and fentanyl, but not agonists specific to non-µ opioid receptors, such as salvinorin A (a k-opioid agonist), nor opioid antagonists or inverse agonists, such as naltrexone (a universal opioid inverse agonist)[10]
- All GABA agonists and positive allosteric modulators of both the GABA-A ionotropic receptor and GABA-B metabotropic receptor subunits, including (partial list):
- alcohol (alcoholic beverage) (cf. alcohol dependence, alcohol withdrawal, delirium tremens)[11]
- barbiturates such as phenobarbital, sodium thiopental and secobarbital
- benzodiazepines such as diazepam (Valium), lorazepam (Ativan), and alprazolam (Xanax) (see benzodiazepine dependence and benzodiazepine withdrawal syndrome)
- nonbenzodiazepine hypnotics (z-drugs) such as zopiclone and zolpidem.[12]
- gamma-hydroxybutyric acid (GHB) and 1,4-butanediol[13]
- carisoprodol (Soma) and related carbamates (tybamate and meprobamate)
- baclofen (Lioresal) and its non-chlorinated analogue phenibut[14]
- chloral hydrate
- glutethimide
- clomethiazole
- methaqualone (Quaalude)
- nicotine (tobacco) (cf. nicotine withdrawal)[15][16]
- gabapentinoids such as gabapentin (Neurontin), pregabalin (Lyrica), and phenibut (Noofen), which are inhibitors of α2δ subunit-containing VDCCs[17][18]
- antiepileptic drugs such as valproate, lamotrigine, tiagabine, vigabatrin, carbamazepine and oxcarbazepine, and topiramate[17][19][20]
- antipsychotic drugs such as clozapine, risperidone, olanzapine, haloperidol, thioridazine, etc.[21]
- commonly prescribed antidepressants such as the selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) (cf. SSRI/SNRI withdrawal syndrome)[22][23][24]
- blood pressure medications, including beta blockers such as propanolol and alpha-adrenergic agonists such as clonidine[25][26]
- androgenic-anabolic steroids[27][28]
- glucocorticoids[29]
Treatment
Treatment for physical dependence depends upon the drug being withdrawn and often includes administration of another drug, especially for substances that can be dangerous when abruptly discontinued or when previous attempts have failed.[30] Physical dependence is usually managed by a slow dose reduction over a period of weeks, months or sometimes longer depending on the drug, dose and the individual.[3] A physical dependence on alcohol is often managed with a cross tolerant drug, such as long acting benzodiazepines to manage the alcohol withdrawal symptoms.
Prognosis
Rebound syndrome
A wide range of drugs whilst not causing a true physical dependence can still cause withdrawal symptoms or rebound effects during dosage reduction or especially abrupt or rapid withdrawal.[31][32] These can include caffeine,[33] stimulants,[34][35][36][37] steroidal drugs and antiparkinsonian drugs.[38] It is debated whether the entire antipsychotic drug class causes true physical dependency, a subset, or if none do.[39] But, if discontinued too rapidly, it could cause an acute withdrawal syndrome.[40] When talking about illicit drugs rebound withdrawal, especially with stimulants, it is sometimes referred to as "coming down" or "crashing".
Some drugs, like anticonvulsants and antidepressants, describe the drug category and not the mechanism. The individual agents and drug classes in the anticonvulsant drug category act at many different receptors and it is not possible to generalize their potential for physical dependence or incidence or severity of rebound syndrome as a group so they must be looked at individually. Anticonvulsants as a group however are known to cause tolerance to the anti-seizure effect.[41] SSRI drugs, which have an important use as antidepressants, engender a discontinuation syndrome that manifests with physical side effects; e.g., there have been case reports of a discontinuation syndrome with venlafaxine (Effexor).[24]
See also
References
- ↑ "Definition of physical dependence - NCI Dictionary of Cancer Terms". 2011-02-02. Archived from the original on 2015-05-10. Retrieved 2015-02-18.
- ↑ "All about Addiction". Medical News Today. Archived from the original on 2018-09-16. Retrieved 2015-02-18.
- 1 2 Landry MJ, Smith DE, McDuff DR, Baughman OL (1992). "Benzodiazepine dependence and withdrawal: identification and medical management". J Am Board Fam Pract. 5 (2): 167–75. PMID 1575069.
- ↑ "Withdrawal From Antidepressants: Symptoms, Causes, Treatments". WebMD. Archived from the original on 2016-02-19. Retrieved 2016-02-20.
These symptoms are not technically the same thing as physical "withdrawal" from a drug.... Unlike drug withdrawal, antidepressant discontinuation effects are not related to addiction but can reflect physiological consequences of stopping a drug, just as when someone with diabetes stops insulin.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–375. ISBN 9780071481274.
- ↑ Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues in Clinical Neuroscience. 15 (4): 431–443. PMC 3898681. PMID 24459410.
Despite the importance of numerous psychosocial factors, at its core, drug addiction involves a biological process: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type [nucleus accumbens] neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement ... Another ΔFosB target is cFos: as ΔFosB accumulates with repeated drug exposure it represses c-Fos and contributes to the molecular switch whereby ΔFosB is selectively induced in the chronic drug-treated state.41. ... Moreover, there is increasing evidence that, despite a range of genetic risks for addiction across the population, exposure to sufficiently high doses of a drug for long periods of time can transform someone who has relatively lower genetic loading into an addict.
- ↑ "Glossary of Terms". Mount Sinai School of Medicine. Department of Neuroscience. Retrieved 9 February 2015.
- ↑ Volkow ND, Koob GF, McLellan AT (January 2016). "Neurobiologic Advances from the Brain Disease Model of Addiction". New England Journal of Medicine. 374 (4): 363–371. doi:10.1056/NEJMra1511480. PMC 6135257. PMID 26816013.
Substance-use disorder: A diagnostic term in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) referring to recurrent use of alcohol or other drugs that causes clinically and functionally significant impairment, such as health problems, disability, and failure to meet major responsibilities at work, school, or home. Depending on the level of severity, this disorder is classified as mild, moderate, or severe.
Addiction: A term used to indicate the most severe, chronic stage of substance-use disorder, in which there is a substantial loss of self-control, as indicated by compulsive drug taking despite the desire to stop taking the drug. In the DSM-5, the term addiction is synonymous with the classification of severe substance-use disorder. - ↑ "Drug addiction (substance use disorder)". Mayo Clinic. Archived from the original on 11 December 2018. Retrieved 4 October 2020.
- ↑ Trang T, Sutak M, Quirion R, Jhamandas K (May 2002). "The role of spinal neuropeptides and prostaglandins in opioid physical dependence". Br. J. Pharmacol. 136 (1): 37–48. doi:10.1038/sj.bjp.0704681. PMC 1762111. PMID 11976266.
- ↑ Kozell L, Belknap JK, Hofstetter JR, Mayeda A, Buck KJ (July 2008). "Mapping a locus for alcohol physical dependence and associated withdrawal to a 1.1 Mb interval of mouse chromosome 1 syntenic with human chromosome 1q23.2-23.3". Genes, Brain and Behavior. 7 (5): 560–7. doi:10.1111/j.1601-183X.2008.00391.x. PMID 18363856.
- ↑ Sikdar S; Ayonrinde, O.; Sampson, E. (July 1998). "Physical dependence on zopiclone. Prescribing this drug to addicts may give rise to iatrogenic drug misuse". BMJ. 317 (7151): 146. doi:10.1136/bmj.317.7151.146. PMC 1113504. PMID 9657802.
- ↑ Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup SA, Smith DE (January 1997). "Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence". Addiction. 92 (1): 89–96. doi:10.1111/j.1360-0443.1997.tb03640.x. PMID 9060200.
- ↑ Alvis, Bret D.; Sobey, Christopher M. (January 2017). "Oral Baclofen Withdrawal Resulting in Progressive Weakness and Sedation Requiring Intensive Care Admission". The Neurohospitalist. 7 (1): 39–40. doi:10.1177/1941874416637404. ISSN 1941-8744. PMC 5167087. PMID 28042369.
- ↑ Kawazoe, Shingo; Shinkai, Takahiro (September 2015). "[Nicotine dependence]". Nihon Rinsho. Japanese Journal of Clinical Medicine. 73 (9): 1516–1521. ISSN 0047-1852. PMID 26394514.
- ↑ Baker, Timothy B.; Piper, Megan E.; Schlam, Tanya R.; Cook, Jessica W.; Smith, Stevens S.; Loh, Wei-Yin; Bolt, Daniel (November 2012). "Are Tobacco Dependence and Withdrawal Related Amongst Heavy Smokers? Relevance to Conceptualizations of Dependence". Journal of Abnormal Psychology. 121 (4): 909–921. doi:10.1037/a0027889. ISSN 0021-843X. PMC 3560396. PMID 22642839.
- 1 2 Tran KT; Hranicky D; Lark T; Jacob Nj (June 2005). "Gabapentin withdrawal syndrome in the presence of a taper". Bipolar Disord. 7 (3): 302–4. doi:10.1111/j.1399-5618.2005.00200.x. PMID 15898970.
- ↑ Weingarten (2019). "Acute phenibut withdrawal: A comprehensive literature review and illustrative case report". Bosnian Journal of Basic Medical Sciences. Department of Anesthesiology and Perioperative Medicine, Mayo Clinic. 19 (2): 125–129. PMC 6535394. PMID 30501608.
- ↑ Hennessy MJ, Tighe MG, Binnie CD, Nashef L (November 2001). "Sudden withdrawal of carbamazepine increases cardiac sympathetic activity in sleep". Neurology. 57 (9): 1650–4. doi:10.1212/WNL.57.9.1650. PMID 11706106. S2CID 22885837.
- ↑ Lazarova M, Petkova B, Staneva-Stoycheva D (December 1999). "Effects of the calcium antagonists verapamil and nitrendipine on carbamazepine withdrawal". Methods Find Exp Clin Pharmacol. 21 (10): 669–71. doi:10.1358/mf.1999.21.10.795757. PMID 10702963. Archived from the original on 2022-04-01. Retrieved 2022-01-26.
- ↑ Meyer, Jonathan M. (January 2011). "Pharmacotherapy of Psychosis and Mania". Goodman and Gilman's The Pharmacological Basis of Therapeutics, Twelfth Edition (12 ed.). McGraw-Hill Education / Medical. p. 435. ISBN 9780071624428.
- ↑ Kora K, Kaplan P (2008). "[Hypomania/mania induced by cessation of antidepressant drugs]". Turk Psikiyatri Derg (in Türkçe). 19 (3): 329–33. PMID 18791886. Archived from the original on 2020-08-06. Retrieved 2022-01-26.
- ↑ Tint A, Haddad PM, Anderson IM (May 2008). "The effect of rate of antidepressant tapering on the incidence of discontinuation symptoms: a randomised study". J. Psychopharmacol. (Oxford). 22 (3): 330–2. doi:10.1177/0269881107087488. PMID 18515448.
- 1 2 Quaglio G, Schifano F, Lugoboni F (September 2008). "Venlafaxine dependence in a patient with a history of alcohol and amineptine misuse". Addiction. 103 (9): 1572–4. doi:10.1111/j.1360-0443.2008.02266.x. PMID 18636997.
- ↑ "MedlinePlus Medical Encyclopedia: Drug abuse and dependence". Archived from the original on 2008-12-07. Retrieved 2008-12-21.
- ↑ Karachalios GN, Charalabopoulos A, Papalimneou V, et al. (May 2005). "Withdrawal syndrome following cessation of antihypertensive drug therapy". Int. J. Clin. Pract. 59 (5): 562–70. doi:10.1111/j.1368-5031.2005.00520.x. PMID 15857353. S2CID 31449302.
- ↑ Trenton AJ, Currier GW (2005). "Behavioural manifestations of anabolic steroid use". CNS Drugs. 19 (7): 571–95. doi:10.2165/00023210-200519070-00002. PMID 15984895. S2CID 32243658.
- ↑ Hartgens F, Kuipers H (2004). "Effects of androgenic-anabolic steroids in athletes". Sports Med. 34 (8): 513–54. doi:10.2165/00007256-200434080-00003. PMID 15248788. S2CID 15234016.
- ↑ Archived May 19, 2013, at the Wayback Machine
- ↑ Jain, Raka; Majumder, Pradipta; Gupta, Tina (January 2013). "Pharmacological Intervention of Nicotine Dependence". BioMed Research International. 2013: 278392. doi:10.1155/2013/278392. ISSN 2314-6133. PMC 3891736. PMID 24490153.
- ↑ Heh CW, Sramek J, Herrera J, Costa J (July 1988). "Exacerbation of psychosis after discontinuation of carbamazepine treatment". Am J Psychiatry. 145 (7): 878–9. doi:10.1176/ajp.145.7.878. PMID 2898213.
- ↑ Henssler J, Heinz A, Brandt L, Bschor T (May 2019). "Antidepressant Withdrawal and Rebound Phenomena". Deutsches Ärzteblatt Online. 116 (20): 355–361. doi:10.3238/arztebl.2019.0355. PMC 6637660. PMID 31288917.
- ↑ Griffiths RR, Evans SM, Heishman SJ, et al. (December 1990). "Low-dose caffeine physical dependence in humans". J. Pharmacol. Exp. Ther. 255 (3): 1123–32. PMID 2262896. Archived from the original on 2022-11-29. Retrieved 2022-01-26.
- ↑ Lake CR, Quirk RS (December 1984). "CNS stimulants and the look-alike drugs". Psychiatr. Clin. North Am. 7 (4): 689–701. doi:10.1016/S0193-953X(18)30723-8. PMID 6151645.
- ↑ Sarampote CS, Efron LA, Robb AS, Pearl PL, Stein MA (2002). "Can stimulant rebound mimic pediatric bipolar disorder?". J Child Adolesc Psychopharmacol. 12 (1): 63–7. doi:10.1089/10445460252943588. PMID 12014597.
- ↑ Danke F (1975). "[Methylphenidate addiction--Reversal of effect on withdrawal]". Psychiatr Clin (Basel) (in Deutsch). 8 (4): 201–11. PMID 1208893.
- ↑ Cohen D, Leo J, Stanton T, et al. (2002). "A boy who stops taking stimulants for "ADHD": commentaries on a Pediatrics case study". Ethical Hum Sci Serv. 4 (3): 189–209. PMID 15278983.
- ↑ Chichmanian RM, Gustovic P, Spreux A, Baldin B (1993). "[Risk related to withdrawal from non-psychotropic drugs]". Thérapie (in français). 48 (5): 415–9. PMID 8146817.
- ↑ Tierney, Lawrence M.; McPhee, Stephen J.; Papadakis, Maxine A. (2008). Current medical diagnosis & treatment, 2008. McGraw-Hill Medical. p. 916. ISBN 978-0-07-149430-4.
- ↑ BNF; British Medical Journal (2008). "Antipsychotic drugs". UK: British National Formulary. Retrieved 22 December 2008.
- ↑ Wolfgang Löscher; Dieter Schmidt (August 2006). "Experimental and Clinical Evidence for Loss of Effect (Tolerance) during Prolonged Treatment with Antiepileptic Drugs". Epilepsia. 47 (8): 1253–1284. doi:10.1111/j.1528-1167.2006.00607.x. PMID 16922870.
External links
- National Institutes of Health MedlinePlus Encyclopedia Archived 2016-07-05 at the Wayback Machine