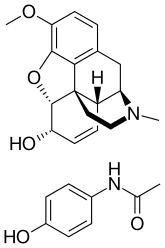
Codeine/paracetamol
 | |
| Combination of | |
|---|---|
| Codeine | Opioid analgesic |
| paracetamol | Anilide analgesic |
| Names | |
| Trade names | Tylenol #3, others |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Legal | |
| Legal status |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ATC code | |
Codeine/paracetamol, also known as codeine/acetaminophen and co-codamol, is a compound analgesic consisting of a combination of codeine phosphate and paracetamol (acetaminophen). Co-codamol tablets are used for the relief of mild to moderate pain when paracetamol or NSAIDs such as ibuprofen, aspirin or naproxen alone do not sufficiently relieve a patient's symptoms, or where their use is ill-advised.
In 2019, it was the 173rd most commonly prescribed medication in the United States, with more than 3 million prescriptions.[1][2]
Medical use
Codeine/paracetamol is a mixture of two painkillers, which are paracetamol and codeine, and can be used for headaches, muscular pain, migraines and toothache.[3]
Formulations
Seven strengths are available:
- 8 mg of codeine phosphate per tablet (e.g. Tylenol 1 in US/Canada)
- 10 mg of codeine phosphate per tablet
- 12.8 mg of codeine phosphate per tablet
- 15 mg of codeine phosphate per tablet (e.g. brands Tylenol 2 in US/Canada, Norway, Australia (multiple brands), United Kingdom)
- 20 mg of codeine phosphate per tablet (Prontalgine in France, Empacod, South Africa and Zimbabwe)
- 30 mg of codeine phosphate per tablet (e.g. Tylenol 3 in US/Canada, ratio-Emtec-30 or "Emtec" in Canada, available elsewhere in capsules such as Tylex or in tablets/caplets e.g. Solpadol, Kapake, Panacod and Zapain).[4]
- 60 mg of codeine phosphate per tablet (e.g. Tylenol 4 in US/Canada, and generally contain from 300 mg to 1000 mg), 1 gram of paracetamol per tablet.[5]
Combination products containing codeine are available over the counter in Barbados, Canada, United Kingdom, Israel and Costa Rica.[6]
Of the European Union (EU) member states, 12 countries (Bulgaria, Cyprus, Denmark, Estonia, France, Ireland, Latvia, Lithuania, Malta, Poland, Romania, Slovenia) allow the sale of OTC codeine solid dosage forms.[7]
In the UK and Ireland the 15/500 and 30/500 tablets are available only with a prescription, although the 8/500 strengths are available over-the-counter. In Australia the 30/500 tablets are available only with a prescription, and the 10/500 and 15/500 tablets were Schedule 3 (Pharmacist Only Medicine) until February 1, 2018 after which they were rescheduled to S4 (prescription only) along with all other codeine products. Manufacturer directions state not to exceed the recommended dosage of two tablets every four hours with a maximum of eight (8 × 500 mg) over a 24-hour period and no more than two (2 × 500 mg) at any one time. Other drugs containing paracetamol must be avoided unless otherwise directed by a prescriber or pharmacist; excessive amounts of paracetamol can lead to serious liver damage. See paracetamol toxicity.
Co-codamol is marketed in Canada and the United States also under the generic name "Atasol Codeine". In the United Kingdom, it is marketed as "Solpadeine Plus" and "Solpadeine Max", as well as "Solpadol". In Australia it is marketed as "Panadeine", "Panadeine Extra" and "Panadeine Forte". In Norway, Co-codamol is sold as "Paralgin Minor" (15/200) (not available as of 2015), "Paralgin Forte" (30/400), "Paralgin Major" (60/800), "Pinex Forte" (30/500) and "Pinex Major" (60/1000).
Side effects
The most common side effects of co-codamol are constipation and feeling sick (nausea) or sleepy.[8] Other side effects may include blood from mouth, skin rashes, dizziness, sedation, shortness of breath, hypersensitivity reaction, fainting (syncope or near syncope), confusion, loss of short-term memory, changes in blood, allergic reactions, euphoria, dysphoria, abdominal pain, itchiness, easy bruising, bleeding gums, vivid dreams, dry mouth and addiction.
Genetic differences between people give rise to differing rates of metabolism of codeine to morphine. In about 5% of people this may happen particularly fast, leading to higher levels of morphine being passed through breast milk in amounts potentially able to cause fatal respiratory depression of a breastfed baby.[9]
See also
References
- ↑ "The Top 300 of 2019". ClinCalc. Archived from the original on 12 February 2021. Retrieved 16 October 2021.
- ↑ "Acetaminophen; Codeine - Drug Usage Statistics". ClinCalc. Archived from the original on 24 October 2021. Retrieved 16 October 2021.
- ↑ "Co-codamol for adults: painkiller containing paracetamol and codeine". nhs.uk. 19 December 2018. Archived from the original on 14 January 2022. Retrieved 24 March 2022.
- ↑ "Tylenol with Codeine #3 Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 2021-08-12. Retrieved 2021-07-24.
- ↑ Tylenol with Codeine Archived 2010-09-13 at the Wayback Machine, from Drugs.com
- ↑ Health risks from codeine based medicines - Whelehan's Pharmacy
- ↑ Bergin M (2015). "The availability of over-the-counter codeine medicines across the European Union". Public Health. 129 (11): 1465–1470. doi:10.1016/j.puhe.2015.06.014. PMID 26215740. Archived from the original on 2022-03-25. Retrieved 2022-02-25.
- ↑ "Co-codamol for adults: painkiller containing paracetamol and codeine - NHS". Https. 19 December 2018. Archived from the original on 14 January 2022. Retrieved April 16, 2021.
- ↑ "Codeine Use While Breastfeeding May Be Dangerous". CTV News. 2008-08-20. Archived from the original on 2008-09-01. Retrieved 2020-08-13.