Monoxerutin
 | |
| Clinical data | |
|---|---|
| Other names | Rutilemone Varemoid Paroven Relvene Monoxerutinum Monoxerutina Monoxerutine |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.729 |
| Chemical and physical data | |
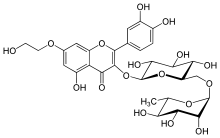
| Formula | C29H34O17 |
| Molar mass | 654.574 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Monoxerutin is a flavonol, a type of flavonoid. It is more accurately a hydroxyethylrutoside.[1]
References
- ↑ Hager H (1994). Hagers Handbuch der pharmazeutischen Praxis (in German). Vol. 8: Stoffe E–O (5th ed.). Springer. ISBN 3-540-52640-4.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.