CTN-986
 | |
| Names | |
|---|---|
| IUPAC name
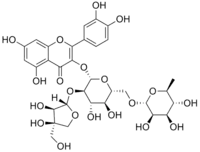
3′,4′,5,7-Tetrahydroxy-3-{[3-C-(hydroxymethyl)-β-D-erythrofuranosyl]-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranosyloxy}flavone | |
| Preferred IUPAC name
(42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-43-{[(2S,3R,4R)-3,4-Dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy}-13,14,25,27,44,45,73,74,75-nonahydroxy-76-methyl-24H-3,6-dioxa-2(2,3)-[1]benzofurana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphane-24-one | |
| Other names
3-[(O-D-apio-β-D-furanosyl-(1→2)-O-[6-deoxy-α-L-mannopyranosyl-(1→6)]-β-D-glucopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one Quercetin 3-O-b-d-apiofuranosyl-(1→2)-[a-l-rhamnopyranosyl-(1→6)]-b-d-glucopyranoside | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C32H38O20 |
| Molar mass | 742.636 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
CTN-986 is a glycoside of quercetin found in cottonseeds and cottonseed oil. In a rodent model, it displays some antidepressant-like properties and stimulation of neurogenesis in the hippocampus.[1] The neurogenesis appears to be mediated by activation of the 5-HT1A receptor, as co-administration with the 5-HT1A antagonist WAY-100,635 abolished the effect.[1]
See also
References
- 1 2 Zhang LM, Zhang YZ, Liu YQ, Gong ZH, Zhao YM, Li YF (2009). "CTN-986, a compound extracted from cottonseeds, increases cell proliferation in hippocampus in vivo and in cultured neural progenitor cells in vitro". Eur J Pharmacol. 607: 110–113. doi:10.1016/j.ejphar.2008.12.052. PMID 19326568.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.