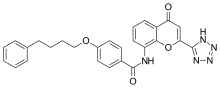
Pranlukast
 | |
| Clinical data | |
|---|---|
| Trade names | Onon (オノン) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic (mainly CYP3A4)[1] |
| Elimination half-life | 1.5 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.236.084 |
| Chemical and physical data | |
| Formula | C27H23N5O4 |
| Molar mass | 481.512 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Pranlukast (brand name Onon, オノン) is a cysteinyl leukotriene receptor-1 antagonist. This drug works similarly to Merck & Co.'s montelukast (Singulair). It is widely used in Japan.
Medications of this class, which go under a variety of names according to whether one looks at the American, British or European system of nomenclature, have as their primary function the antagonism of bronchospasm caused, principally in asthmatics, by an allergic reaction to accidentally or inadvertently encountered allergens.[2]
Medications of this group are normally used as an adjunct to the standard therapy of inhaled steroids with inhaled long- and/or short-acting beta-agonists. There are several similar medications in the group; all appear to be equally effective. Pranlukast is also reported as potential inhibitor of Mycobacterium tuberculosis infection in experimental models.[3]
References
- 1 2 Nakade S, Ueda S, Ohno T, Nakayama K, Miyata Y, Yukawa E, Higuchi S (2006). "Population pharmacokinetics of pranlukast hydrate dry syrup in children with allergic rhinitis and bronchial asthma". Drug Metab Pharmacokinet. 21 (2): 133–9. doi:10.2133/dmpk.21.133. PMID 16702733. Archived from the original on 2008-09-17.
- ↑ Singh, Rakesh Kumar; Tandon, Ruchi; Dastidar, Sunanda Ghosh; Ray, Abhijit (2013). "A review on leukotrienes and their receptors with reference to asthma". Journal of Asthma. 50 (9): 922–931. doi:10.3109/02770903.2013.823447. ISSN 0277-0903. PMID 23859232. S2CID 11433313.
- ↑ Mishra A; et al. (2018). "An allosteric inhibitor of Mycobacterium tuberculosis ArgJ: Implications to a novel combinatorial therapy". EMBO Molecular Medicine. 10 (4): e8038. doi:10.15252/emmm.201708038. PMC 5887547. PMID 29483133.