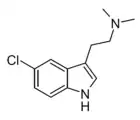
5-Chloro-DMT
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H15ClN2 |
| Molar mass | 222.72 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
5-Chloro-N,N-dimethyltryptamine (5-chloro-DMT) is a tryptamine derivative related to compounds such as 5-bromo-DMT and 5-fluoro-DMT. It acts as a serotonin receptor agonist and has primarily sedative effects in animal studies.[1][2][3] It has been sold as a designer drug.[4]
See also
- 5,N,N-TMT
- 5-Chloro-AMT
- 6-Fluoro-AMT
- 6-Fluoro-DMT
- 7-Chloro-AMT
References
- ↑ Benington F, Morin RD, Clark LC (September 1960). "Synthesis of some 5- and 6-chloro, 5-methyl, and 5,6,7-trimethyl derivatives of tryptamine". Journal of Organic Chemistry. 25 (9): 1542–1547. doi:10.1021/jo01079a020.
- ↑ Ibrahim MA, El-Alfy AT, Ezel K, Radwan MO, Shilabin AG, Kochanowska-Karamyan AJ, et al. (August 2017). "Marine Inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as Modulators of Serotonin Receptors: An Example Illustrating the Power of Bromine as Part of the Uniquely Marine Chemical Space". Marine Drugs. 15 (8). doi:10.3390/md15080248. PMC 5577603. PMID 28792478.
- ↑ Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, et al. (May 2021). "Psychedelic-inspired drug discovery using an engineered biosensor". Cell. 184 (10): 2779–2792.e18. doi:10.1016/j.cell.2021.03.043. PMID 33915107.
- ↑ "Analytical Report: 5-Cl-DMT" (PDF). Slovenia: Nacionalni Forenzični Laboratorij. July 2020.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.