Inotersen
| Names | |
|---|---|
| Trade names | Tegsedi |
| Other names | GSK-2998728, ISIS-420915 |
| Clinical data | |
| Drug class | Antisense oligonucleotide[1] |
| Main uses | Hereditary transthyretin-mediated amyloidosis (hATTR)[1] |
| Side effects | Redness and pain at the site of injection, nausea, headache, tiredness, low platelets, fever[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
| Formula | C230H318N69O121P19S19 |
| Molar mass | 7183.08 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Inotersen, sold under the brand name Tegsedi, is a medication used to treat nerve damage in hereditary transthyretin-mediated amyloidosis (hATTR).[1] It is used in people who are still able to walk.[6] It is given by injection under the skin.[1]
Common side effects redness and pain at the site of injection, nausea, headache, tiredness, low platelets, and fever.[2] Other side effects may include kidney inflammation, stroke, vitamin A deficiency, allergic reactions, and liver problems.[2][1] It is a antisense oligonucleotide.[1]
Inotersen was approved for medical use in the United States and Europe in 2018.[1][6] In the United Kingdom it costs the NHS about £332,000 per year as of 2021.[7] In the United States this amount costs about 521,000 USD while in Canada it is about 420,000 CAD.[8][9]
Medical use
Dosage
It is given at a dose of 284 mg once per week.[7]
Because of these serious side effects, inotersen is available in the United States only through a restricted program called the Tegsedi Risk Evaluation and Mitigation (REMS) Program.[2]
Chemistry
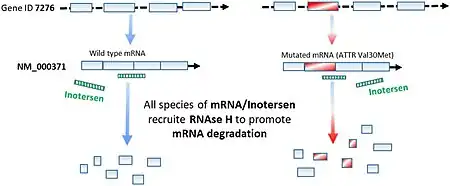
It is a antisense oligonucleotide.[1] The sequence is TCTTG GTTACATGAA ATCCC, where C is methylated C, and the first and third section (bases 1-5 and 16-20, separated from the middle section by spaces) are MOE-modified.[11]
In terms of medication(s) used to treat nerve damage for hereditary transthyretin-mediated amyloidosis the most common means are by transcriptional regulatory therapy which come via RNA interference (or antisense oligonucleotides)[10]
History
Inotersen was approved for medical use in the European Union in July 2018.[5]
The U.S. Food and Drug Administration (FDA) approved inotersen in October 2018.[2] The application for inotersen was granted orphan drug designation.[12]
The FDA approved inotersen based on evidence from one clinical trial (Trial 1/NCT01737398) that included 172 patients with hereditary transthyretin-mediated amyloidosis.[2] The trial was conducted at 24 sites in Australia, Europe, South America, and the United States.[2]
The benefits and side effects of inotersen were evaluated in one clinical trial that enrolled patients with hereditary transthyretin-mediated amyloidosis.[2] Patients were randomly assigned to receive inotersen or placebo by subcutaneous injection given once a week for 65 weeks.[2] During the first week of treatment, patients received three doses of treatment, followed by once weekly subcutaneous injections for 64 weeks.[2] Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.[2]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[13]
References
- 1 2 3 4 5 6 7 8 "Inotersen Monograph for Professionals". Drugs.com. Archived from the original on 4 December 2021. Retrieved 26 November 2021.
- 1 2 3 4 5 6 7 8 9 10 11 "Drug Trial Snapshot: Tegsedi". U.S. Food and Drug Administration (FDA). 23 July 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Tegsedi 284 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)". (emc). 15 October 2019. Archived from the original on 7 October 2020. Retrieved 3 October 2020.
- ↑ "Tegsedi- inotersen injection, solution". DailyMed. 2 September 2020. Archived from the original on 7 October 2020. Retrieved 3 October 2020.
- 1 2 "Tegsedi EPAR". European Medicines Agency (EMA). Archived from the original on 8 October 2020. Retrieved 3 October 2020.
- 1 2 "Tegsedi". Archived from the original on 8 October 2020. Retrieved 26 November 2021.
- 1 2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1109. ISBN 978-0857114105.
- ↑ "Tegsedi Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 November 2021. Retrieved 26 November 2021.
- ↑ "CADTH Canadian Drug Expert Committee Recommendation" (PDF). CADTH. Archived (PDF) from the original on 20 October 2020. Retrieved 26 November 2021.
- 1 2 Barresi, Vincenza; Musmeci, Camillo; Rinaldi, Alessandro; Condorelli, Daniele Filippo (9 August 2022). "Transcript-Targeted Therapy Based on RNA Interference and Antisense Oligonucleotides: Current Applications and Novel Molecular Targets". International Journal of Molecular Sciences. 23 (16): 8875. doi:10.3390/ijms23168875. ISSN 1422-0067. Archived from the original on 26 August 2022. Retrieved 23 October 2023.
- ↑ "Nucleid Acid Ther". Archived from the original on 31 October 2021. Retrieved 26 July 2021.
- ↑ "Inotersen Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). 24 July 2012. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Archived from the original on 17 September 2020. Retrieved 16 September 2020.
External links
| Identifiers: |
|---|
- "Inotersen". Drug Information Portal. U.S. National Library of Medicine (NLM). Archived from the original on 5 May 2021. Retrieved 22 October 2021.