Carcinoid syndrome
| Carcinoid syndrome | |
|---|---|
| Other names: Carcinoid apudoma,[1] functioning argentaffinoma,[1] Thorson-Bioerck syndrome[2] | |
| Video explanation | |
| Specialty | Endocrinology, oncology |
| Symptoms | Flushing, diarrhea, wheezing, tiredness[3] |
| Complications | Low blood pressure, niacin deficiency, valvular heart disease[3] |
| Usual onset | Around 55 to 60 years old[3] |
| Causes | Neuroendocrine tumors[3] |
| Diagnostic method | 24 hour urine 5-HIAA followed by medical imaging to locate the tumor[3] |
| Differential diagnosis | Irritable bowel syndrome, anaphylaxis, celiac disease, Ogilvie syndrome[3] |
| Treatment | Octreotide, lanreotide, surgery, chemotherapy[3] |
| Frequency | 5 per million per year[3] |
Carcinoid syndrome is a group of symptoms that occur as a result of substances released by neuroendocrine tumors (carcinoid tumors).[3] Symptoms may includes flushing, diarrhea, wheezing, itchiness, muscle wasting, and tiredness.[3] Complications may include low blood pressure, niacin deficiency, and valvular heart disease, most commonly tricuspid insufficiency.[3][4]
It occurs in about 10% of people with neuroendocrine tumors.[3] Most commonly these are tumors that involve the midgut and spread to the liver.[3] Attacks may be triggered by eating, alcohol, or stress.[3] The mechanism involve substances such as serotonin, histamine, tachykinins, kallikrein, and prostaglandins.[3] It is classified as a paraneoplastic syndrome.[5] Diagnosis is generally by 24 hour urine 5-HIAA followed by medical imaging to locate the tumor.[3]
Treatment may include octreotide, lanreotide, surgery, and chemotherapy.[3] Carcinoid syndrome is rare.[3] About 5 per million people are newly affected a year.[3] Onsets is most often around 55 to 60 years old.[3] Males and females are effected nearly equally.[3] The condition was first described in 1953.[6]
Signs and symptoms
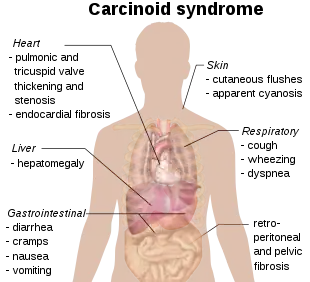
The carcinoid syndrome occurs in approximately 5% of carcinoid tumors[8] and becomes manifest when vasoactive substances from the tumors enter the systemic circulation escaping hepatic degradation. If the primary tumor is from the gastrointestinal tract (hence releasing serotonin into the hepatic portal circulation), carcinoid syndrome generally does not occur until the disease is so advanced that it overwhelms the liver's ability to metabolize the released serotonin.
- Flushing: The most important clinical finding is flushing of the skin, usually of the head and the upper part of thorax.[9][10]
- Diarrhea: When the diarrhea is intense and explosive, it may lead to electrolyte disturbance and dehydration.
- Abdominal pain: Due to desmoplastic reaction of the mesentery or hepatic metastases.
- Bronchoconstriction, which may be histamine-induced, affects a smaller number of patients and often accompanies flushing.
- Secondary restrictive cardiomyopathy: About 50% of patients have cardiac abnormalities classically of the restrictive-type caused by serotonin-induced fibrosis of the valvular endocardium, notably the tricuspid and pulmonary valves, called cardiac fibrosis. This results in a heart with normal rhythm and contractility, but reduced preload and end-diastolic volume. "TIPS" is an acronym for Tricuspid Insufficiency, Pulmonary Stenosis (fibrosis of tricuspid and pulmonary valves).
- Nausea and vomiting
Pathophysiology
Carcinoid tumors produce several vasoactive substances, most prominently serotonin. It is commonly thought that serotonin is the cause of the flushing, but this is only partially correct.[11] The flushing also results from secretion of kallikrein, the enzyme that catalyzes the conversion of kininogen to lysyl-bradykinin. The latter is further converted to bradykinin, one of the most powerful vasodilators known.
Other components of the carcinoid syndrome are diarrhea (probably caused by the increased serotonin, which greatly increases peristalsis, leaving less time for fluid absorption), a pellagra-like syndrome (probably caused by diversion of large amounts of tryptophan from synthesis of the vitamin B3 niacin, which is needed for NAD production, to the synthesis of serotonin and other 5-hydroxyindoles), fibrotic lesions of the endocardium, particularly on the right side of the heart resulting in insufficiency of the tricuspid valve and, less frequently, the pulmonary valve and, uncommonly, bronchoconstriction.
The pathogenesis of the cardiac lesions and the bronchoconstriction is unknown, but the former probably involves activation of serotonin 5-HT2B receptors by serotonin. When the primary tumor is in the gastrointestinal tract, as it is in the great majority of cases, the serotonin and kallikrein are inactivated in the liver; manifestations of carcinoid syndrome do not occur until there are metastases to the liver or when the cancer is accompanied by liver failure (cirrhosis). Carcinoid tumors arising in the bronchi may be associated with manifestations of carcinoid syndrome without liver metastases because their biologically active products reach the systemic circulation before passing through the liver and being metabolized.
In most patients, there is an increased urinary excretion of 5-HIAA (5-hydroxyindoleacetic acid), a degradation product of serotonin.
The biology of these tumors differs from many other tumor types. Ongoing research on the biology of these tumors may reveal new mechanisms for tumor development.[12]
Diagnosis

With a certain degree of clinical suspicion, the most useful initial test is the 24-hour urine levels of 5-HIAA (5-hydroxyindoleacetic acid), the end product of serotonin metabolism. Patients with carcinoid syndrome usually excrete more than 25 mg of 5-HIAA per day.
Imaging
For localization of both primary lesions and metastasis, the initial imaging method is Octreoscan, where indium-111 labelled somatostatin analogues (octreotide) are used in scintigraphy for detecting tumors expressing somatostatin receptors. Median detection rates with octreoscan are about 89%, in contrast to other imaging techniques such as CT scan and MRI with detection rates of about 80%. Gallium-68 labelled somatostatin analogues such as 68Ga-DOTA-Octreotate (DOTATATE), performed on a PET/CT scanner is superior to conventional Octreoscan.
Usually, on a CT scan, a spider-like/crab-like change is visible in the mesentery due to the fibrosis from the release of serotonin. 18F-FDG PET/CT, which evaluate for increased metabolism of glucose, may also aid in localizing the carcinoid lesion or evaluating for metastases. Chromogranin A and platelets serotonin are increased.
Localization of tumour
Tumour localization may be extremely difficult. Barium swallow and follow-up examination of the intestine may occasionally show the tumor. Capsule video endoscopy has recently been used to localize the tumor. Often laparotomy is the definitive way to localize the tumour. Another form of localizing a tumor is the Octreoscan. A tracer agent of Indium 111 is injected into a vein where then the tumors absorb the radionuclide Indium 111 and become visible on the scanner. Only the tumors absorb the somatostatin agent Indium 111 making the scan highly effective.
Treatment
For symptomatic relief of carcinoid syndrome:
- Octreotide (a somatostatin analogue which decreases the secretion of serotonin by the tumor and, secondarily, decreases the breakdown product of serotonin (5-HIAA))
- Telotristat ethyl along with a somatostatin analogue in patients not responding to somatostatin analogue monotherapy.[13] It is a tryptophan hydroxylase inhibitor and reduces the production of serotonin.[14]
- Peptide receptor radionuclide therapy (PRRT) with lutetium-177, yttrium-90 or indium-111 labeled to octreotate is highly effective
- Methysergide maleate (antiserotonin agent but not used because of the serious side effect of retroperitoneal fibrosis)
- Cyproheptadine (an antihistamine drug with antiserotonergic effects)
Alternative treatment for qualifying candidates:
- Surgical resection of tumor and chemotherapy (5-FU and doxorubicin)
- Endovascular, chemoembolization, targeted chemotherapy directly delivered to the liver through special catheters mixed with embolic beads (particles that block blood vessels), used for patients with liver metastases.
Uncertainties
Disease progression is difficult to ascertain because the disease can metastasize anywhere in the body and can be too small to identify with any current technology. Markers of the condition such as chromogranin-A are imperfect indicators of disease progression.[15]
Prognosis
Prognosis varies from individual to individual. It ranges from a 95% 5-year survival for localized disease to an 80% 5-year survival for those with liver metastases. The average survival time from the start of octreotide treatment has increased to about 12 years.
See also
References
- 1 2 "Carcinoid Syndrome". NORD (National Organization for Rare Disorders). Archived from the original on 14 June 2020. Retrieved 8 May 2019.
- ↑ Cote, Etienne (2010). Clinical Veterinary Advisor - E-Book: An Excerpt from Clinical Veterinary Advisor 2e - Reprint. Elsevier Health Sciences. p. 1732. ISBN 9780323068765. Archived from the original on 2020-07-11. Retrieved 2019-05-08.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Pandit, S; Annamaraju, P; Bhusal, K (January 2020). "Carcinoid Syndrome". PMID 28846309.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Kilickapp, Saadettin; Yalcin, Suayib (2015). "29. Carcinoid syndrome". In Yalcin, Suayib; Öberg, Kjell (eds.). Neuroendocrine Tumours: Diagnosis and Management. Heidelberg: Springer. p. 515. ISBN 978-3-662-45214-1. Archived from the original on 2022-02-06. Retrieved 2022-02-11.
- ↑ Shields, Thomas W.; LoCicero, Joseph; Reed, Carolyn E.; Feins, Richard H. (2009). General Thoracic Surgery. Lippincott Williams & Wilkins. p. 1540. ISBN 978-0-7817-7982-1. Archived from the original on 2021-08-29. Retrieved 2020-12-25.
- ↑ Norton, Jeffrey; Bollinger, R. Randall; Chang, Alfred E.; Lowry, Stephen F. (2012). Surgery: Basic Science and Clinical Evidence. Springer. p. 852. ISBN 978-3-642-57282-1. Archived from the original on 2021-08-29. Retrieved 2020-12-25.
- ↑ Table 15-14 in: Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. ISBN 978-1-4160-2973-1.
- ↑ Warrell; et al. (2010). Oxford Textbook of Medicine (8th ed.). Oxford University Press. ISBN 978-0-19-262922-7.
- ↑ E.Goljan, Pathology, 2nd ed Mosby Elsevier, Rapid Review series.
- ↑ Soga J, Yakuwa Y, Osaka M (1999). "Carcinoid syndrome: a statistical evaluation of 748 reported cases". Journal of Experimental & Clinical Cancer Research. 18 (2): 133–41. PMID 10464698.
- ↑ Papaliodis, Dean, et al. "Niacin-induced “flush” involves release of prostaglandin D2 from mast cells and serotonin from platelets: evidence from human cells in vitro and an animal model." Journal of Pharmacology and Experimental Therapeutics 327.3 (2008): 665-672.
- ↑ Cunningham JL, Janson ET (2011). "The biological hallmarks of ileal carcinoids". European Journal of Clinical Investigation. 41 (12): 1353–60. doi:10.1111/j.1365-2362.2011.02537.x. PMID 21605115.
- ↑ "FDA Approves Xermelo for Carcinoid Syndrome Diarrhea". U.S. Food and Drug Administration. February 28, 2017. Archived from the original on 1 March 2017. Retrieved 1 March 2017.
- ↑ "Xermelo (telotristat ethyl) Tablets, for Oral Use. Full Prescribing Information" (PDF). Lexicon Pharmaceuticals, Inc. 8800 Technology Forest Place. The Woodlands, TX 77381. Archived (PDF) from the original on 1 July 2017. Retrieved 1 March 2017.
- ↑ Nobels FR, Kwekkeboom DJ, Bouillon R, Lamberts SW (1998). "Chromogranin A: its clinical value as marker of neuroendocrine tumours". European Journal of Clinical Investigation. 28 (6): 431–40. doi:10.1046/j.1365-2362.1998.00305.x. PMID 9693933.
External links
- Mabvuure N, Cumberworth A, Hindocha S (2012). "In patients with carcinoid syndrome undergoing valve replacement: will a biological valve have acceptable durability?". Interactive Cardiovascular and Thoracic Surgery. 15 (3): 467–71. doi:10.1093/icvts/ivs212. PMC 3422934. PMID 22691379.
| Classification | |
|---|---|
| External resources |
.svg.png.webp)