Hepatitis C virus
| Hepacivirus C | |
|---|---|
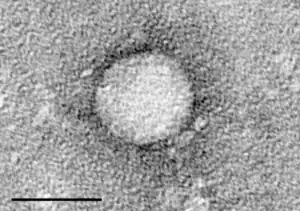 | |
| Electron micrograph of Hepacivirus C purified from cell culture. Scale bar = 50 nanometres | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Kitrinoviricota |
| Class: | Flasuviricetes |
| Order: | Amarillovirales |
| Family: | Flaviviridae |
| Genus: | Hepacivirus |
| Species: | Hepacivirus C |
| Synonyms[1][2] | |
|
Hepatitis C virus | |
The hepatitis C virus (HCV[3]) is a small (55–65 nm in size), enveloped, positive-sense single-stranded RNA virus of the family Flaviviridae.[4]
The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer (hepatocellular carcinoma, abbreviated HCC) and lymphomas in humans.[5][6]
In 2017 , the WHO indicates that 71 million individuals were infected with the virus[7]
In 2020 the Nobel Prize in Medicine was awarded for the discovery of Hepatitis C virus to Harvey J. Alter, Michael Houghton and Charles M. Rice[8]
Classification
The hepatitis C virus belongs to the genus Hepacivirus, a member of the family Flaviviridae. Before 2011, it was considered to be the only member of this genus. However a member of this genus has been discovered in dogs: canine hepacivirus.[9] There is also at least one virus in this genus that infects horses.[10] Several additional viruses in the genus have been described in bats and rodents.[11][12]
Structure

The hepatitis C virus particle consists of a lipid membrane envelope that is 55 to 65 nm in diameter.[13][14] Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope.[15] They take part in viral attachment and entry into the cell.[13] Within the envelope is an icosahedral core that is 33 to 40 nm in diameter.[14] Inside the core is the RNA material of the virus.[13]
E1 and E2 glycoproteins
E1 and E2 are covalently bonded when embedded in the envelope of HCV and are stabilized by disulfide bonds. E2 is globular and seems to protrude 6 nm out from the envelope membrane according to electron microscope images.[14]
These glycoproteins play an important role in the interactions hepatitis C has with the immune system. A hypervariable region, the hypervariable region 1 (HVR1) can be found on the E2 glycoprotein.[13] HVR1 is flexible and quite accessible to surrounding molecules.[16] HVR1 helps E2 shield the virus from the immune system. It prevents CD81 from latching onto its respective receptor on the virus.[16] In addition, E2 can shield E1 from the immune system.[16] Although HVR1 is quite variable in amino acid sequence, this region has similar chemical, physical, and conformational characteristics across many E2 glycoproteins.[17]
Genome
Hepatitis C virus has a positive sense single-stranded RNA genome. The genome consists of a single open reading frame that is 9,600 nucleotide bases long.[18] This single open reading frame is translated to produce a single protein product, which is then further processed to produce smaller active proteins. This is why on publicly available databases, such as the European Bioinformatics Institute, the viral proteome only consists of 2 proteins.[19][20]
At the 5′ and 3′ ends of the RNA are the untranslated regions (UTR), that are not translated into proteins but are important to translation and replication of the viral RNA. The 5′ UTR has a ribosome binding site[21] or internal ribosome entry site (IRES) that initiates the translation of a very long protein containing about 3,000 amino acids. The core domain of the HCV IRES contains a four-way helical Holliday junction that is integrated within a predicted pseudoknot.[22] The conformation of this core domain constrains the open reading frame's orientation for positioning on the 40S ribosomal subunit. The large pre-protein is later cleaved by cellular and viral proteases into the 10 smaller proteins that allow viral replication within the host cell, or assemble into the mature viral particles.[23] Structural proteins made by the hepatitis C virus include Core protein, E1 and E2; nonstructural proteins include NS2, NS3, NS4A, NS4B, NS5A, and NS5B.[19][24]
Molecular biology
The proteins of this virus are arranged along the genome in the following order: N terminal-core-envelope (E1)–E2–p7-nonstructural protein 2 (NS2)–NS3–NS4A–NS4B–NS5A–NS5B–C terminal. The mature nonstructural proteins (NS2 to NS5B) generation relies on the activity of viral proteinases.[25] The NS2/NS3 junction is cleaved by a metal-dependent autocatalytic proteinase encoded within NS2 and the N-terminus of NS3. The remaining cleavages downstream from this site are catalysed by a serine protease also contained within the N-terminal region of NS3.[26][27]
- The core protein has 191 amino acids and can be divided into three domains on the basis of hydrophobicity: domain 1 (residues 1–117) contains mainly basic residues with two short hydrophobic regions; domain 2 (residues 118–174) is less basic and more hydrophobic and its C-terminus is at the end of p21; domain 3 (residues 175–191) is highly hydrophobic and acts as a signal sequence for E1 envelope protein.[28][29][30][31]
- Both envelope proteins (E1 and E2) are highly glycosylated and important in cell entry. E1 serves as the fusogenic subunit and E2 acts as the receptor binding protein. E1 has 4–5 N-linked glycans and E2 has 11 N-glycosylation sites.[32][33][34]
- NS1 (p7) protein is dispensable for viral genome replication but plays a critical role in virus morphogenesis. This protein is a 63 amino acid membrane-spanning protein which locates itself in the endoplasmic reticulum. Cleavage of p7 is mediated by the endoplasmic reticulum's signal peptidases. Two transmembrane domains of p7 are connected by a cytoplasmic loop and are oriented towards the endoplasmic reticulum's lumen.[35][36][37]
- NS2 protein is a 21–23 kiloDalton (kDa) transmembrane protein with protease activity.[27][38]
- NS3 is 67 kDa protein whose N-terminal has serine protease activity and whose C-terminal has NTPase/helicase activity. It is located within the endoplasmic reticulum and forms a heterodimeric complex with NS4A—a 54 amino acid membrane protein that acts as a cofactor of the proteinase.[39][38]
- NS4A—a 54 amino acid membrane protein that acts as a cofactor of the proteinase.[40]
- NS4B is a small (27 kDa) hydrophobic integral membrane protein with four transmembrane domains. It is located within the endoplasmic reticulum and plays an important role for recruitment of other viral proteins. It induces morphological changes to the endoplasmic reticulum forming a structure termed the membranous web.[41][42]
- NS5A is a hydrophilic phosphoprotein which plays an important role in viral replication, modulation of cell signaling pathways and the interferon response. It is known to bind to endoplasmic reticulum-anchored human VAP proteins.[43]
- The NS5B protein (65 kDa) is the viral RNA-dependent RNA polymerase. NS5B has the key function of replicating the HCV's viral RNA by using the viral positive sense RNA strand as its template and catalyzes the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication.[44][45][46] Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK (HCV-BK, genotype 1).[47] The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4's (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication.[48] Current research attempts to bind structures to this active site to alter its functionality in order to prevent further viral RNA replication.[49]
An 11th protein has also been described, This protein is encoded by a +1 frameshift in the capsid gene, it appears to be antigenic but its function is unknown.[50][51]
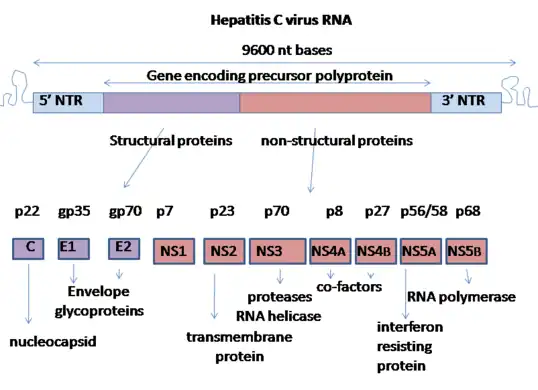 Genome organisation of hepatitis C virus
Genome organisation of hepatitis C virus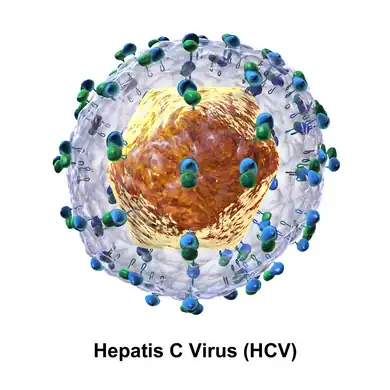 Diagram of the structure of the hepatitis C virus particle
Diagram of the structure of the hepatitis C virus particle
Replication
Replication of HCV involves several steps. The virus replicates mainly in the hepatocytes of the liver, where it is estimated that daily each infected cell produces approximately fifty virions (virus particles) with a calculated total of one trillion virions generated. The virus may also replicate in peripheral blood mononuclear cells, potentially accounting for the high levels of immunological disorders found in chronically infected HCV patients. In the liver, the HCV particles are brought into the hepatic sinusoids by blood flow. These sinusoids neighbor hepatocyte cells.[13] HCV is able to pass through the endothelium of the sinusoids and make its way to the basolateral surface of the hepatocyte cells.[13]
HCV has a wide variety of genotypes and mutates rapidly due to a high error rate on the part of the virus' RNA-dependent RNA polymerase. The mutation rate produces so many variants of the virus it is considered a quasispecies rather than a conventional virus species.[52] Entry into host cells occur through complex interactions between virions, especially through their glycoproteins, and cell-surface molecules CD81, LDL receptor, SR-BI, DC-SIGN, Claudin-1, and Occludin.[53][54]
The envelope of HCV is similar to very low-density lipoproteins (VLDL) and low-density lipoproteins (LDL).[13] Because of this similarity, the virus is thought to be able to associate with apolipoproteins. It could surround itself with lipoproteins, partially covering up E1 and E2. Recent research indicates that these apolipoproteins interact with scavenger receptor B1 (SR-B1). SR-B1 is able to remove lipids from the lipoproteins around the virus to better allow for HVR1 contact. Claudin 1, which is a tight-junction protein, and CD81 link to create a complex, priming them for later HCV infection processes. As the immune system is triggered, macrophages increase the amount of TNF-α around the hepatocytes which are being infected. This triggers the migration of occludin, which is another tight-junction complex, to the basolateral membrane. The HCV particle is ready to enter the cell.[13]
These interactions lead to the endocytosis of the viral particle. This process is aided by clathrin proteins. Once inside an early endosome, the endosome and the viral envelope fuse and the RNA is allowed into the cytoplasm.[13]
HCV takes over portions of the intracellular machinery to replicate.[55] The HCV genome is translated to produce a single protein of around 3,011 amino acids. The polyprotein is then proteolytically processed by viral and cellular proteases to produce three structural (virion-associated) and seven nonstructural (NS) proteins. Alternatively, a frameshift may occur in the Core region to produce an alternate reading frame protein (ARFP).[56]
HCV encodes two proteases, the NS2 cysteine autoprotease and the NS3-4A serine protease; the NS proteins then recruit the viral genome into an RNA replication complex, which is associated with rearranged cytoplasmic membranes. RNA replication takes place via the viral RNA-dependent RNA polymerase NS5B, which produces a negative strand RNA intermediate. The negative strand RNA then serves as a template for the production of new positive strand viral genomes. Nascent genomes can then be translated, further replicated or packaged within new virus particles.[27][57]
The virus replicates on intracellular lipid membranes.[58] The endoplasmic reticulum in particular is deformed into uniquely shaped membrane structures termed 'membranous webs'. These structures can be induced by sole expression of the viral protein NS4B.[59] The core protein associates with lipid droplets and utilises microtubules and dyneins to alter their location to a perinuclear distribution.[60] Release from the hepatocyte may involve the VLDL secretory pathway.[61] Another hypothesis states that the viral particle may be secreted from the endoplasmic reticulum through the endosomal sorting complex required for transport (ESCRT) pathway.[13] This pathway is normally utilized to bud vesicles out of the cell. The only limitation to this hypothesis is that the pathway is normally used for cellular budding, and it is not known how HCV would commandeer the ESCRT pathway for use with the endoplasmic reticulum.[13]
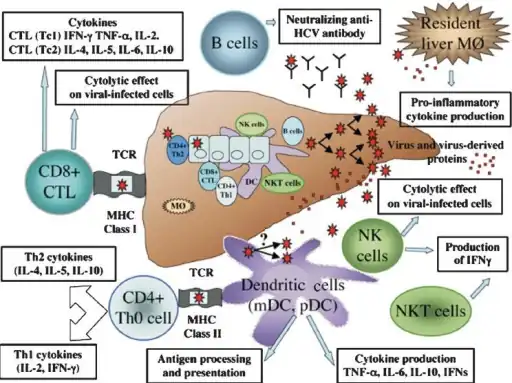 Immune response in viral hepatitis C involves both the innate and adaptive immune system
Immune response in viral hepatitis C involves both the innate and adaptive immune system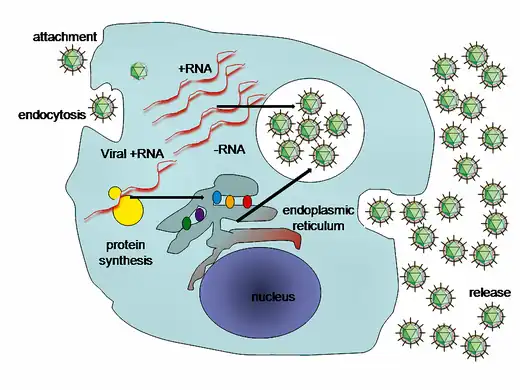 A simplified diagram of the hepatitis C virus replication cycle
A simplified diagram of the hepatitis C virus replication cycle
Genotypes

Based on genetic differences between HCV isolates, the hepatitis C virus species is classified into six genotypes (1–6) with several subtypes within each genotype (represented by lowercase letters).[62][63] Subtypes are further broken down into quasispecies based on their genetic diversity. Genotypes differ by 30–35% of the nucleotide sites over the complete genome.[64] The difference in genomic composition of subtypes of a genotype is usually low[65]. Subtypes 1a and 1b are found worldwide and cause 60% of all cases.[66]
Clinical importance
Genotype is clinically important in determining potential response to interferon-based therapy and the required duration of such therapy. Genotypes 1 and 4 are less responsive to interferon-based treatment than are the other genotypes (2, 3, 5 and 6).[67] The duration of standard interferon-based therapy for genotypes 1 and 4 is 48 weeks, whereas treatment for genotypes 2 and 3 is completed in 24 weeks. Sustained virological responses occur in 70% of genotype 1 cases, ~90% of genotypes 2 and 3, ~65% of genotype 4 and ~80% of genotype 6.[68] In addition, people of African descent are much less likely to respond to treatment when infected with genotypes 1 or 4.[69] The substantial proportion of this lack of response to treatment is proposed to be caused by a single-nucleotide polymorphism (SNP) on chromosome 19 of the human genome that is predictive of treatment success.[70] HCV genotypes 1 and 4 have been distributed endemically in overlapping areas of West and Central Africa, infecting for centuries human populations carrying the genetic polymorphism in question. This has prompted scientists to suggest that the protracted persistence of HCV genotypes 1 and 4 in people of African origin is an evolutionary adaptation of HCV over many centuries to these populations’ immunogenetic responses.[71]
Infection with one genotype does not confer immunity against others, and concurrent infection with two strains is possible. In most of these cases, one of the strains outcompetes the other in a short time. This finding may be useful in treatment, in replacing strains non-responsive to medication with others easier to treat.[72]
Recombination
When two viruses infect the same cell, genetic recombination may occur.[73] Although infrequent, HCV recombination has been observed between different genotypes, between subtypes of the same genotype and even between strains of the same subtype.[73]
Evolution
Identification of the origin of this virus has been difficult but genotypes 1 and 4 appear to share a common origin.[74] A Bayesian analysis suggests that the major genotypes diverged about 300–400 years ago from the common ancestor virus.The minor genotypes diverged about 200 years ago from their major genotypes. All of the extant genotypes appear to have evolved from genotype 1 subtype 1b.[75][76]
A study of genotype 6 strains suggests an earlier date of evolution: approximately 1,100 to 1,350 years Before Present.[77] The estimated rate of mutation was 1.8 × 10−4. An experimental study estimated the mutation rate at 2.5–2.9 × 10−3 base substitutions per site per year.[78] This genotype may be the ancestor of the other genotypes.[77]
A study of European, US and Japanese isolates suggested that the date of origin of genotype 1b was approximately in the year 1925. The estimated dates of origin of types 2a and 3a were 1917 and 1943 respectively. The time of divergence of types 1a and 1b was estimated to be 200–300 years.[79]
A study of genotype 1a and 1b estimated the dates of origin to be the early 20th century. Both types 1a and 1b underwent massive expansions in their effective population size between 1940 and 1980. The expansion of HCV subtype 1b preceded that of subtype 1a by at least a decade. Both types appear to have spread from the developed world to the developing world.[66]
The genotype 2 strains from Africa can be divided into four clades that correlate with their country of origin: (1) Cameroon and Central African Republic (2) Benin, Ghana and Burkina Faso (3) Gambia, Guinea, Guinea-Bissau and Senegal (4) Madagascar.[80] There is also strong evidence for the dissemination of HCV genotype 2 from West Africa to the Caribbean by the trans-Atlantic slave trade.[81]
Genotype 3 is thought to have its origin in South East Asia.[82]
These dates from these various countries suggests that this virus may have evolved in South East Asia and was spread to West Africa by traders from Western Europe.[83] It was later introduced into Japan ( once that country's self-imposed isolation was lifted[84]), once introduced to a country its spread has been influenced by many local factors including blood transfusions, vaccination programs and other means. Given the reduction in the rate of spread once screening for HCV in blood products was implemented in the 1990s, it would seem that previously blood transfusion was an important method of spread.[85][86][87]
Disease
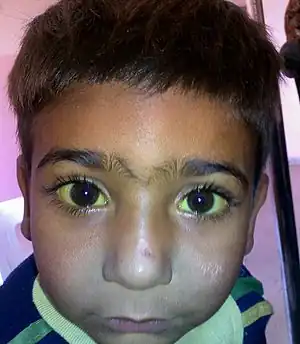
Hepatitis C is an infectious disease caused by the hepatitis C virus that primarily affects the liver.[88] During the initial infection people often have mild or no symptoms.[89] Occasionally a fever, dark urine, abdominal pain, and yellow tinged skin occurs.[89] The virus persists in the liver in about 75% to 85% of those initially infected.[89] Early on chronic infection typically has no symptoms.[89] Over many years however, it often leads to liver disease and occasionally cirrhosis.[89] In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.[88]
Prevention
Unlike hepatitis A and B, there is currently no vaccine to prevent hepatitis C infection.[90]
Treatment
Chronic infection can be cured at a very high percentage most of the time; this is done via antiviral medications[7]
Epidemiology

Hepatitis C virus is predominantly a blood-borne virus, with very low risk of sexual or vertical transmission.[91]
Because of this mode of spread the key groups at risk are intravenous drug users (IDUs), recipients of blood products and sometimes patients on haemodialysis. Common setting for transmission of HCV is also intra-hospital (nosocomial) transmission, when practices of hygiene and sterilization are not correctly followed in the clinic.[92]
A number of cultural or ritual practices have been proposed as a potential historical mode of spread for HCV, including circumcision, genital mutilation, ritual scarification, traditional tattooing and acupuncture.[91]
It has also been argued that given the extremely prolonged periods of persistence of HCV in humans, even very low and undetectable rates of mechanical transmission via biting insects may be sufficient to maintain endemic infection in the tropics, where people receive large number of insect bites.[93]
Current research


Scientific work done in 1988, among Michael Houghton, Charles M. Rice and Harvey Alter led to the discovery of the Hepatitis C virus and in 2020 the Nobel prize in Medicine.[94]: 194 [8]
The study of HCV has been hampered by the narrow host range of HCV.[95] The use of replicons has been successful but these have only been recently discovered.[96] HCV, as with most RNA viruses, exists as a viral quasispecies, making it very difficult to isolate a single strain or receptor type for study.[97][98]
Current research is focused on small-molecule inhibitors of the viral protease, RNA polymerase and other nonstructural genes. Two agents—boceprevir and telaprevir —both inhibitors of NS3 protease were approved for use on May 13, 2011 and May 23, 2011 respectively.[99]
A possible association between low Vitamin D levels and a poor response to treatment has been reported.[100][101][102][103] In vitro work has shown that vitamin D may be able to reduce viral replication.[104] While this work looks promising[105][106] the results of clinical trials are pending.[107][108] However, it has been proposed that vitamin D supplementation is important in addition to standard treatment, in order to enhance treatment response.[109]
Naringenin, a flavonoid found in grapefruit and other fruits and herbs, has been shown to block the assembly of intracellular infectious viral particles without affecting intracellular levels of the viral RNA or protein.[109]
Other agents that are under investigation include nucleoside and nucleotide analogue inhibitors and non-nucleoside inhibitors of the RNA-dependent RNA polymerase, inhibitors of NSP5A, and host-targeted compounds such as cyclophilin inhibitors and silibinin.[110]
Sofosbuvir for use against chronic hepatitis C infection was approved by the FDA on December 6, 2013. It has been reported to be the first drug that has demonstrated safety and efficacy to treat certain types of HCV infection without the need for co-administration of interferon.[111] On November 22, the FDA approved simeprevir for use in combination with peginterferon-alfa and ribavirin.[112] Simeprevir has been approved in Japan for the treatment of chronic hepatitis C infection, genotype 1.[113]
There is also current experimental research on non drug related therapies. Oxymatrine, for example, is a root extract found in the continent of Asia that has been reported to have antiviral activity against HCV in cell cultures and animal studies. Small and promising human trials have shown beneficial results and no serious side effects, but they were too small to generalize conclusions.[109]
See also
- Blood-borne disease
- Cancer virus
- Discovery and development of NS5A inhibitors
- HCV IRES
- Hepatitis C virus stem-loop VII
- Hepatitis C virus 3′X element
- Hepatitis C virus (HCV) cis-acting replication element (CRE)
- Sexually transmitted infection
References
- ↑ "Hepacivirus C". National Center for Biotechnology Information (NCBI). Retrieved 3 January 2022.
- ↑ Smith, Donald B.; et al. (23 June 2016). "Create 13 new species in the genus Hepacivirusand rename 1 species (family Flaviviridae)" (PDF). International Committee on Taxonomy of Viruses (ICTV). Archived (PDF) from the original on 24 October 2022. Retrieved 13 March 2019.
- ↑ Basit, Hajira; Tyagi, Isha; Koirala, Janak (2023). "Hepatitis C". StatPearls. StatPearls Publishing. PMID 28613647. Archived from the original on 2022-05-20. Retrieved 2023-08-29.
- ↑ "Genus: Hepacivirus". International Committee on Taxonomy of Viruses. July 2018. Archived from the original on February 19, 2020.
- ↑ Ferri, Clodoveo (2015). "HCV syndrome: A constellation of organ- and non-organ specific autoimmune disorders, B-cell non-Hodgkin's lymphoma, and cancer". World Journal of Hepatology. 7 (3): 327–43. doi:10.4254/wjh.v7.i3.327. ISSN 1948-5182. PMC 4381161. PMID 25848462.
- ↑ Rusyn I, Lemon SM (2014). "Mechanisms of HCV-induced liver cancer: what did we learn from in vitro and animal studies?". Cancer Lett. 345 (2): 210–5. doi:10.1016/j.canlet.2013.06.028. PMC 3844040. PMID 23871966.
- 1 2 "Hepatitis C". 26 May 2020. Archived from the original on 26 May 2020. Retrieved 31 August 2023.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - 1 2 "The Nobel Prize in Physiology or Medicine 2020". NobelPrize.org. Archived from the original on 27 March 2023. Retrieved 1 September 2023.
- ↑ Kapoor A, et al. (2011). "Characterization of a canine homolog of hepatitis C virus". Proc Natl Acad Sci U S A. 108 (28): 11608–13. Bibcode:2011PNAS..10811608K. doi:10.1073/pnas.1101794108. PMC 3136326. PMID 21610165.
- ↑ Burbelo PD, Dubovi EJ, Simmonds P, et al. (June 2012). "Serology-enabled discovery of genetically diverse hepaciviruses in a new host". J. Virol. 86 (11): 6171–8. doi:10.1128/JVI.00250-12. PMC 3372197. PMID 22491452.
- ↑ Quan PL, Firth C, Conte JM, et al. (May 2013). "Bats are a major natural reservoir for hepaciviruses and pegiviruses". Proc. Natl. Acad. Sci. U.S.A. 110 (20): 8194–9. Bibcode:2013PNAS..110.8194Q. doi:10.1073/pnas.1303037110. PMC 3657805. PMID 23610427.
- ↑ Kapoor A, Simmonds P, Scheel TK, et al. (2013). "Identification of rodent homologs of hepatitis C virus and pegiviruses". mBio. 4 (2): e00216–13. doi:10.1128/mBio.00216-13. PMC 3622934. PMID 23572554.
- 1 2 3 4 5 6 7 8 9 10 11 Dubuisson, Jean; Cosset, François-Loïc (2014). "Virology and cell biology of the hepatitis C virus life cycle – An update". Journal of Hepatology. 61 (1): S3–S13. doi:10.1016/j.jhep.2014.06.031. PMID 25443344.
- 1 2 3 Kaito, Masahiko; Ishida, Satoshi; Tanaka, Hideaki; Horiike, Shinichiro; Fujita, Naoki; Adachi, Yukihiko; Kohara, Michinori; Konishi, Masayoshi; Watanabe, Shozo (June 2006). "Morphology of hepatitis C and hepatitis B virus particles as detected by immunogold electron microscopy". Medical Molecular Morphology. 39 (2): 63–71. doi:10.1007/s00795-006-0317-8. ISSN 1860-1480. PMID 16821143. S2CID 24668769.
- ↑ Op De Beeck A, Dubuisson J (2003). "Topology of hepatitis C virus envelope glycoproteins". Rev. Med. Virol. 13 (4): 233–41. doi:10.1002/rmv.391. PMID 12820185. S2CID 22280227.
- 1 2 3 Castelli, Matteo; Clementi, Nicola; Pfaff, Jennifer; Sautto, Giuseppe A.; Diotti, Roberta A.; Burioni, Roberto; Doranz, Benjamin J.; Dal Peraro, Matteo; Clementi, Massimo (2017-03-16). "A Biologically-validated HCV E1E2 Heterodimer Structural Model". Scientific Reports. 7 (1): 214. Bibcode:2017NatSR...7..214C. doi:10.1038/s41598-017-00320-7. ISSN 2045-2322. PMC 5428263. PMID 28303031.
- ↑ Basu, Arnab; Beyene, Aster; Meyer, Keith; Ray, Ranjit (May 2004). "The Hypervariable Region 1 of the E2 Glycoprotein of Hepatitis C Virus Binds to Glycosaminoglycans, but This Binding Does Not Lead to Infection in a Pseudotype System". Journal of Virology. 78 (9): 4478–4486. doi:10.1128/JVI.78.9.4478-4486.2004. ISSN 0022-538X. PMC 387685. PMID 15078928.
- ↑ Kato N (2000). "Genome of human hepatitis C virus (HCV): gene organization, sequence diversity, and variation". Microb. Comp. Genom. 5 (3): 129–51. doi:10.1089/mcg.2000.5.129. PMID 11252351.
- 1 2 Chevaliez, Stéphane; Pawlotsky, Jean-Michel (2006). "HCV Genome and Life Cycle". Hepatitis C Viruses: Genomes and Molecular Biology. Horizon Bioscience. ISBN 9781904933205. PMID 21250393. Archived from the original on 25 June 2022. Retrieved 31 August 2023.
- ↑ Institute, European Bioinformatics. "EBI Search". ebi.ac.uk. Archived from the original on 7 September 2023. Retrieved 5 September 2023.
- ↑ Jubin R (2001). "Hepatitis C IRES: translating translation into a therapeutic target". Curr. Opin. Mol. Ther. 3 (3): 278–87. PMID 11497352.
- ↑ Berry KE, Waghray S, Mortimer SA, Bai Y, Doudna JA (October 2011). "Crystal structure of the HCV IRES central domain reveals strategy for start-codon positioning". Structure. 19 (10): 1456–66. doi:10.1016/j.str.2011.08.002. PMC 3209822. PMID 22000514.
- ↑ Dubuisson J (2007). "Hepatitis C virus proteins". World J. Gastroenterol. 13 (17): 2406–15. doi:10.3748/wjg.v13.i17.2406. PMC 4146758. PMID 17552023.
- ↑ Jarnagin, William R. (13 September 2022). Blumgart's Surgery of the Liver, Biliary Tract and Pancreas, 2-Volume Set - E-Book. Elsevier Health Sciences. p. 142. ISBN 978-0-323-69785-9. Archived from the original on 10 September 2023. Retrieved 9 September 2023.
- ↑ De Francesco R (1999). "Molecular virology of the hepatitis C virus". J Hepatol. 31 (Suppl 1): 47–53. doi:10.1016/S0168-8278(99)80374-2. PMID 10622560.
- ↑ Wang, Bo; Meng, Xiang-Jin (2021). "Structural and molecular biology of hepatitis E virus". Computational and Structural Biotechnology Journal. 19: 1907–1916. doi:10.1016/j.csbj.2021.03.038. ISSN 2001-0370. Archived from the original on 8 September 2022. Retrieved 1 September 2023.
- 1 2 3 Welbourn, Sarah; Pause, Arnim (2006). "HCV NS2/3 Protease". Hepatitis C Viruses: Genomes and Molecular Biology. Horizon Bioscience. ISBN 978-1-904933-20-5. Archived from the original on 2023-09-04. Retrieved 2023-09-01.
- ↑ Hagedorn, C. H.; Rice, C. M. (6 December 2012). The Hepatitis C Viruses. Springer Science & Business Media. p. 119. ISBN 978-3-642-59605-6. Archived from the original on 4 September 2023. Retrieved 2 September 2023.
- ↑ Mani, H; Yen, JH; Hsu, HJ; Chang, CC; Liou, JW (April 2022). "Hepatitis C virus core protein: Not just a nucleocapsid building block, but an immunity and inflammation modulator". Tzu chi medical journal. 34 (2): 139–147. doi:10.4103/tcmj.tcmj_97_21. PMID 35465281.
- ↑ Gawlik, Katarzyna; Gallay, Philippe A. (October 2014). "HCV core protein and virus assembly: what we know without structures". Immunologic research. 60 (1): 1–10. doi:10.1007/s12026-014-8494-3. ISSN 0257-277X. Archived from the original on 16 September 2023. Retrieved 13 September 2023.
- ↑ Boulant, Steeve; Vanbelle, Christophe; Ebel, Christine; Penin, François; Lavergne, Jean-Pierre (September 2005). "Hepatitis C virus core protein is a dimeric alpha-helical protein exhibiting membrane protein features". Journal of Virology. 79 (17): 11353–11365. doi:10.1128/JVI.79.17.11353-11365.2005. ISSN 0022-538X. Archived from the original on 21 June 2022. Retrieved 13 September 2023.
- ↑ Tong, Yimin; Lavillette, Dimitri; Li, Qingchao; Zhong, Jin (19 June 2018). "Role of Hepatitis C Virus Envelope Glycoprotein E1 in Virus Entry and Assembly". Frontiers in Immunology. 9: 1411. doi:10.3389/fimmu.2018.01411. ISSN 1664-3224. Archived from the original on 4 September 2023. Retrieved 2 September 2023.
- ↑ Lavie, Muriel; Hanoulle, Xavier; Dubuisson, Jean (2018). "Glycan Shielding and Modulation of Hepatitis C Virus Neutralizing Antibodies". Frontiers in Immunology. 9: 910. doi:10.3389/fimmu.2018.00910. ISSN 1664-3224. Archived from the original on 6 November 2022. Retrieved 3 September 2023.
- ↑ Torrents de la Peña, Alba; Sliepen, Kwinten; Eshun-Wilson, Lisa; Newby, Maddy L.; Allen, Joel D.; Zon, Ian; Koekkoek, Sylvie; Chumbe, Ana; Crispin, Max; Schinkel, Janke; Lander, Gabriel C.; Sanders, Rogier W.; Ward, Andrew B. (21 October 2022). "Structure of the hepatitis C virus E1E2 glycoprotein complex". Science (New York, N.Y.). 378 (6617): 263–269. doi:10.1126/science.abn9884. ISSN 1095-9203. Archived from the original on 20 July 2023. Retrieved 10 September 2023.
- ↑ Taniguchi, S.; Okamoto, H.; Sakamoto, M.; Kojima, M.; Tsuda, F.; Tanaka, T.; Munekata, E.; Muchmore, E. E.; Peterson, D. A.; Mishiro, S. (July 1993). "A structurally flexible and antigenically variable N-terminal domain of the hepatitis C virus E2/NS1 protein: implication for an escape from antibody". Virology. 195 (1): 297–301. doi:10.1006/viro.1993.1378. ISSN 0042-6822. Archived from the original on 20 June 2022. Retrieved 2 September 2023.
- ↑ Steinmann, Eike; Penin, Francois; Kallis, Stephanie; Patel, Arvind H.; Bartenschlager, Ralf; Pietschmann, Thomas (July 2007). "Hepatitis C virus p7 protein is crucial for assembly and release of infectious virions". PLoS pathogens. 3 (7): e103. doi:10.1371/journal.ppat.0030103. ISSN 1553-7374. Archived from the original on 14 August 2022. Retrieved 8 September 2023.
- ↑ Kalita, Monoj Mon; Griffin, Stephen; Chou, James J.; Fischer, Wolfgang B. (June 2015). "Genotype-specific differences in structural features of hepatitis C virus (HCV) p7 membrane protein". Biochimica Et Biophysica Acta. 1848 (6): 1383–1392. doi:10.1016/j.bbamem.2015.03.006. ISSN 0006-3002. Archived from the original on 2022-06-15. Retrieved 2023-09-16.
- 1 2 Ashfaq, Usman A.; Javed, Tariq; Rehman, Sidra; Nawaz, Zafar; Riazuddin, Sheikh (11 April 2011). "An overview of HCV molecular biology, replication and immune responses". Virology Journal. 8 (1): 161. doi:10.1186/1743-422X-8-161. ISSN 1743-422X. Archived from the original on 6 June 2023. Retrieved 4 September 2023.
- ↑ Raney, Kevin D.; Sharma, Suresh D.; Moustafa, Ibrahim M.; Cameron, Craig E. (23 July 2010). "Hepatitis C virus non-structural protein 3 (HCV NS3): a multifunctional antiviral target". The Journal of Biological Chemistry. 285 (30): 22725–22731. doi:10.1074/jbc.R110.125294. ISSN 1083-351X. Archived from the original on 13 March 2023. Retrieved 1 September 2023.
- ↑ Phan, Tung; Kohlway, Andrew; Dimberu, Peniel; Pyle, Anna Marie; Lindenbach, Brett D. (2011). "The Acidic Domain of Hepatitis C Virus NS4A Contributes to RNA Replication and Virus Particle Assembly". Journal of Virology. 85 (3): 1193–1204. doi:10.1128/JVI.01889-10. ISSN 0022-538X. Archived from the original on 4 September 2023. Retrieved 2 September 2023.
- ↑ Sklan, Ella H.; Glenn, Jeffrey S. (2006). "HCV NS4B: From Obscurity to Central Stage". Hepatitis C Viruses: Genomes and Molecular Biology. Horizon Bioscience. ISBN 978-1-904933-20-5. Archived from the original on 2023-09-04. Retrieved 2023-09-01.
- ↑ Jiang, XH; Xie, YT; Cai, YP; Ren, J; Ma, T (25 May 2017). "Effects of hepatitis C virus core protein and nonstructural protein 4B on the Wnt/β-catenin pathway". BMC microbiology. 17 (1): 124. doi:10.1186/s12866-017-1032-4. PMID 28545480. Archived from the original on 7 September 2023. Retrieved 4 September 2023.
- ↑ Gupta G, Qin H, Song J (2012). "Intrinsically unstructured domain 3 of hepatitis C Virus NS5A forms a "fuzzy complex" with VAPB-MSP domain which carries ALS-causing mutations". PLOS ONE. 7 (6): e39261. Bibcode:2012PLoSO...739261G. doi:10.1371/journal.pone.0039261. PMC 3374797. PMID 22720086.
- ↑ Jin, Z; Leveque, V; Ma, H; Johnson, K. A.; Klumpp, K (2012). "Assembly, purification, and pre-steady-state kinetic analysis of active RNA-dependent RNA polymerase elongation complex". Journal of Biological Chemistry. 287 (13): 10674–83. doi:10.1074/jbc.M111.325530. PMC 3323022. PMID 22303022.
- ↑ Moradpour D, Penin F, Rice CM (June 2007). "Replication of hepatitis C virus". Nat. Rev. Microbiol. 5 (6): 453–63. doi:10.1038/nrmicro1645. PMID 17487147. S2CID 13176201.
- ↑ Rigat K, Wang Y, Hudyma TW, et al. (November 2010). "Ligand-induced changes in hepatitis C virus NS5B polymerase structure". Antiviral Res. 88 (2): 197–206. doi:10.1016/j.antiviral.2010.08.014. PMID 20813137.
- ↑ Biswal BK, Cherney MM, Wang M, et al. (May 2005). "Crystal structures of the RNA-dependent RNA polymerase genotype 2a of hepatitis C virus reveal two conformations and suggest mechanisms of inhibition by non-nucleoside inhibitors". J. Biol. Chem. 280 (18): 18202–10. doi:10.1074/jbc.M413410200. PMID 15746101.
- ↑ O'Farrell D, Trowbridge R, Rowlands D, Jäger J (February 2003). "Substrate complexes of hepatitis C virus RNA polymerase (HC-J4): structural evidence for nucleotide import and de-novo initiation". J. Mol. Biol. 326 (4): 1025–35. doi:10.1016/s0022-2836(02)01439-0. PMID 12589751.
- ↑ Biswal BK, Wang M, Cherney MM, et al. (August 2006). "Non-nucleoside inhibitors binding to hepatitis C virus NS5B polymerase reveal a novel mechanism of inhibition". J. Mol. Biol. 361 (1): 33–45. doi:10.1016/j.jmb.2006.05.074. PMID 16828488.
- ↑ Walewski JL, Keller TR, Stump DD, Branch AD (2001). "Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame". RNA. 7 (5): 710–721. doi:10.1017/S1355838201010111. PMC 1370123. PMID 11350035.
- ↑ Baghbani-arani F, Roohvand F, Aghasadeghi MR, Eidi A, Amini S, Motevalli F, Sadat SM, Memarnejadian A, Khalili G, et al. (2012). "Expression and characterization of Escherichia coli derived hepatitis C virus ARFP/F protein". Mol Biol (Mosk). 46 (2): 251–9. doi:10.1134/S0026893312020033. PMID 22670521. S2CID 7379944.
- ↑ Bartenschlager R, Lohmann V (July 2000). "Replication of hepatitis C virus". J. Gen. Virol. 81 (Pt 7): 1631–48. CiteSeerX 10.1.1.319.8775. doi:10.1099/0022-1317-81-7-1631. PMID 10859368. Archived from the original on 2009-12-03. Retrieved 2010-07-16.
- ↑ Zeisel, M.; Barth, H.; Schuster, C.; Baumert, T. (2009). "Hepatitis C virus entry: molecular mechanisms and targets for antiviral therapy". Frontiers in Bioscience. 14 (8): 3274–3285. Bibcode:2009CNSNS..14.3274H. doi:10.1016/j.cnsns.2008.11.006. PMC 3235086. PMID 19273272.
- ↑ Kohaar, I.; Ploss, A.; Korol, E.; Mu, K.; Schoggins, J.; O'Brien, T.; Rice, C.; Prokunina-Olsson, L. (2010). "Splicing diversity of the human OCLN gene and its biological significance for hepatitis C virus entry". Journal of Virology. 84 (14): 6987–6994. doi:10.1128/JVI.00196-10. PMC 2898237. PMID 20463075.
- ↑ Lindenbach B, Rice C (2005). "Unravelling hepatitis C virus replication from genome to function". Nature. 436 (7053): 933–8. Bibcode:2005Natur.436..933L. doi:10.1038/nature04077. PMID 16107832.
- ↑ Branch, A. D.; Stump, D. D.; Gutierrez, J. A.; Eng, F.; Walewski, J. L. (2005). "The Hepatitis C Virus Alternate Reading Frame (ARF) and Its Family of Novel Products: The Alternate Reading Frame Protein/F-Protein, the Double-Frameshift Protein, and Others". Seminars in Liver Disease. 25 (1): 105–117. doi:10.1055/s-2005-864786. PMID 15732002.
- ↑ Shu, Bo; Gong, Peng (12 July 2016). "Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation". Proceedings of the National Academy of Sciences. 113 (28). doi:10.1073/pnas.1602591113. ISSN 0027-8424. Archived from the original on 30 January 2023. Retrieved 5 September 2023.
- ↑ Dubuisson J, Penin F, Moradpour D (2002). "Interaction of hepatitis C virus proteins with host cell membranes and lipids". Trends Cell Biol. 12 (11): 517–523. doi:10.1016/S0962-8924(02)02383-8. PMID 12446113.
- ↑ Egger D, Wölk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K (2002). "Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex". J Virol. 76 (12): 5974–84. doi:10.1128/JVI.76.12.5974-5984.2002. PMC 136238. PMID 12021330.
- ↑ Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J (2008). "Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner". Traffic. 9 (8): 1268–82. doi:10.1111/j.1600-0854.2008.00767.x. PMID 18489704. S2CID 20609887.
- ↑ Syed GH, Amako Y, Siddiqui A (2010). "Hepatitis C virus hijacks host lipid metabolism". Trends Endocrinol Metab. 21 (1): 33–40. doi:10.1016/j.tem.2009.07.005. PMC 2818172. PMID 19854061.
- ↑ Simmonds P, Holmes EC, Cha TA, et al. (November 1993). "Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region" (PDF). J. Gen. Virol. 74 (Pt 11): 2391–9. CiteSeerX 10.1.1.325.7888. doi:10.1099/0022-1317-74-11-2391. PMID 8245854. S2CID 17597460. Archived from the original on 1 July 2023. Retrieved 10 July 2020.
- ↑ Nakano, Tatsunori; Lau, Gillian M. G.; Lau, Grace M. L.; Sugiyama, Masaya; Mizokami, Masashi (9 October 2011). "An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region". Liver International. 32 (2): 339–45. doi:10.1111/j.1478-3231.2011.02684.x. PMID 22142261. S2CID 23271017.
- ↑ Ohno O, Mizokami M, Wu RR, Saleh MG, Ohba K, Orito E, Mukaide M, Williams R, Lau JY, et al. (2007). "New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a". J Clin Microbiol. 35 (1): 201–7. doi:10.1128/JCM.35.1.201-207.1997. PMC 229539. PMID 8968908.
- ↑ Manso, Carmen F.; Bibby, David F.; Lythgow, Kieren; Mohamed, Hodan; Myers, Richard; Williams, David; Piorkowska, Renata; Chan, Yuen T.; Bowden, Rory; Ansari, M. Azim; Ip, Camilla L. C.; Barnes, Eleanor; Bradshaw, Daniel; Mbisa, Jean L. (2020). "Technical Validation of a Hepatitis C Virus Whole Genome Sequencing Assay for Detection of Genotype and Antiviral Resistance in the Clinical Pathway". Frontiers in Microbiology. 11. doi:10.3389/fmicb.2020.576572/full. ISSN 1664-302X. Archived from the original on 25 October 2022. Retrieved 14 September 2023.
- 1 2 Magiorkinis, Gkikas; Magiorkinis, Emmanouil; Paraskevis, Dimitrios; Ho, Simon Y. W.; Shapiro, Beth; Pybus, Oliver G.; Allain, Jean-Pierre; Hatzakis, Angelos (December 2009). "The global spread of hepatitis C virus 1a and 1b: a phylodynamic and phylogeographic analysis". PLoS medicine. 6 (12): e1000198. doi:10.1371/journal.pmed.1000198. ISSN 1549-1676. Archived from the original on 22 April 2023. Retrieved 5 September 2023.
- ↑ Simmonds P; Bukh J; Combet C; Deléage G; Enomoto N; Feinstone S; Halfon P; Inchauspé G; Kuiken C; Maertens G; Mizokami M; Murphy, DG; Okamoto, H; Pawlotsky, JM; Penin, F; Sablon, E; Shin-I, T; Stuyver, LJ; Thiel, HJ; Viazov, S; Weiner, AJ; Widell, A (2005). "Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes". Hepatology. 42 (4): 962–73. doi:10.1002/hep.20819. PMID 16149085. S2CID 21393716.
- ↑ Yu ML, Chuang WL (2009). "Treatment of chronic hepatitis C in Asia: when East meets West". J Gastroenterol Hepatol. 24 (3): 336–345. doi:10.1111/j.1440-1746.2009.05789.x. PMID 19335784. S2CID 27333980.
- ↑ Muir, AJ; Bornstein, JD; Killenberg, PG; Atlantic Coast Hepatitis Treatment Group (2004). "Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites". N Engl J Med. 350 (22): 2265–71. doi:10.1056/NEJMoa032502. PMID 15163776. Erratum: doi:10.1056/nejm200409163511229
- ↑ Ge, D; Fellay, J; Thompson, AJ; Simon, SJ; Shianna, KV; Urban, TJ; Heinzen, EL; et al. (2009). "Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance". Nature. 461 (7262): 399–401. Bibcode:2009Natur.461..399G. doi:10.1038/nature08309. PMID 19684573. S2CID 1707096.
- ↑ Rose, R; Markov, PV; Lam, TT; Pybus, OG (2013). "Viral evolution explains the associations among hepatitis C virus genotype, clinical outcomes, and human genetic variation". Infect Genet Evol. 20: 418–21. doi:10.1016/j.meegid.2013.09.029. hdl:10722/221827. PMID 24140473.
- ↑ Laskus T, Wang LF, Radkowski M, Vargas H, Nowicki M, Wilkinson J, Rakela J (2001). "Exposure of hepatitis C virus (HCV) RNA-positive recipients to HCV RNA-positive blood donors results in rapid predominance of a single donor strain and exclusion and/or suppression of the recipient strain". Journal of Virology. 75 (5): 2059–66. doi:10.1128/JVI.75.5.2059-2066.2001. PMC 114790. PMID 11160710.
- 1 2 González-Candelas F, López-Labrador FX, Bracho MA (Oct 2011). "Recombination in hepatitis C virus". Viruses. 3 (10): 2006–24. doi:10.3390/v3102006. PMC 3205392. PMID 22069526.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Salemi M, Vandamme AM (2002). "Hepatitis C virus evolutionary patterns studied through analysis of full-genome sequences". J Mol Evol. 54 (1): 62–70. Bibcode:2002JMolE..54...62S. doi:10.1007/s00239-001-0018-9. PMID 11734899. S2CID 35899454.
- ↑ Sarwar MT, et al. (2011). "NS4A protein as a marker of HCV history suggests that different HCV genotypes originally evolved from genotype 1b". Virol. J. 8: 317. doi:10.1186/1743-422X-8-317. PMC 3145594. PMID 21696641.
- ↑ Forni, Diego; Cagliani, Rachele; Pontremoli, Chiara; Pozzoli, Uberto; Vertemara, Jacopo; De Gioia, Luca; Clerici, Mario; Sironi, Manuela (2018). "Evolutionary Analysis Provides Insight Into the Origin and Adaptation of HCV". Frontiers in Microbiology. 9: 854. doi:10.3389/fmicb.2018.00854. ISSN 1664-302X. Archived from the original on 22 November 2022. Retrieved 6 September 2023.
- 1 2 Pybus OG, Barnes E, Taggart R, Lemey P, Markov PV, Rasachak B, Syhavong B, Phetsouvanah R, Sheridan I, et al. (2009). "Genetic history of hepatitis C virus in East Asia". J Virol. 83 (2): 1071–82. doi:10.1128/JVI.01501-08. PMC 2612398. PMID 18971279.
- ↑ Kato N, Ueda Y, Sejima H, Gu W, Satoh S, Dansako H, Ikeda M, Shimotohno K (2019) Study of multiple genetic variations caused by persistent hepatitis C virus replication in long-term cell culture. Arch Virol
- ↑ Simmonds P, Smith DB (1997). "Investigation of the pattern of diversity of hepatitis C virus in relation to times of transmission". Journal of Viral Hepatitis. 4 (Suppl 1): 69–74. doi:10.1111/j.1365-2893.1997.tb00163.x. PMID 9097281. S2CID 41594303.
- ↑ Markov PV, Pepin J, Frost E, Deslandes S, Labbé AC, Pybus OG (September 2009). "Phylogeography and molecular epidemiology of hepatitis C virus genotype 2 in Africa". J. Gen. Virol. 90 (Pt 9): 2086–96. doi:10.1099/vir.0.011569-0. PMID 19474244.
- ↑ Markov, PV; van de Laar, TJ; Thomas, XV; Aronson, SJ; Weegink, CJ; van den Berk, GE; Prins, M.; et al. (2012). "Colonial History and Contemporary Transmission Shape the Genetic Diversity of Hepatitis C Virus Genotype 2 in Amsterdam". J Virol. 86 (14): 7677–7687. doi:10.1128/JVI.06910-11. PMC 3416291. PMID 22573865.
- ↑ Simmonds P (November 2004). "Genetic diversity and evolution of hepatitis C virus—15 years on". J. Gen. Virol. 85 (Pt 11): 3173–88. doi:10.1099/vir.0.80401-0. PMID 15483230.
- ↑ Simmonds P (2001). "Reconstructing the origins of human hepatitis viruses". Philos Trans R Soc Lond B Biol Sci. 356 (1411): 1013–26. doi:10.1098/rstb.2001.0890. PMC 1088496. PMID 11516379.
- ↑ Furukawa, Ryuzo; Suto, Yuko; Ishida, Emile H.; Yamauchi, Takeshi (5 February 2019). Lifestyle and Nature: Integrating Nature Technology to Sustainable Lifestyles. CRC Press. p. 73. ISBN 978-1-351-37837-6. Archived from the original on 16 September 2023. Retrieved 15 September 2023. Japan's isolation from the outside world was completed with the fifth order issued ... Edo period was an era, unique in world history, which saw no war for 265 years (1603–1868)
- ↑ Bukh, Jens (October 2016). "The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control". Journal of Hepatology. 65 (1 Suppl): S2–S21. doi:10.1016/j.jhep.2016.07.035. ISSN 1600-0641. Archived from the original on 14 June 2023. Retrieved 3 September 2023.
- ↑ Yatsuhashi, Hiroshi (2016). "Past, Present, and Future of Viral Hepatitis C in Japan". Euroasian Journal of Hepato-Gastroenterology. 6 (1): 49–51. doi:10.5005/jp-journals-10018-1166. ISSN 2231-5047. Archived from the original on 10 September 2023. Retrieved 8 September 2023.
- ↑ Prati, Daniele (October 2006). "Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review". Journal of Hepatology. 45 (4): 607–616. doi:10.1016/j.jhep.2006.07.003. ISSN 0168-8278. Archived from the original on 21 March 2023. Retrieved 15 September 2023.
- 1 2 Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 551–52. ISBN 978-0-8385-8529-0.
- 1 2 3 4 5 "Hepatitis C Questions and Answers for Health Professionals | CDC". www.cdc.gov. 9 December 2022. Archived from the original on 21 January 2016. Retrieved 31 August 2023.
- ↑ Yu CI, Chiang BL (2010). "A new insight into hepatitis C vaccine development". J. Biomed. Biotechnol. 2010: 1–12. doi:10.1155/2010/548280. PMC 2896694. PMID 20625493.
- 1 2 Shepard, CW; Finelli, L; Alter, MJ (Sep 2005). "Global epidemiology of hepatitis C virus infection". Lancet Infect Dis. 5 (9): 558–67. doi:10.1016/S1473-3099(05)70216-4. PMID 16122679. Archived from the original on 2023-04-22. Retrieved 2023-01-30.
- ↑ Alter, MJ (Nov 2011). "HCV routes of transmission: what goes around comes around". Semin Liver Dis. 31 (4): 340–6. doi:10.1055/s-0031-1297923. PMID 22189974.
- ↑ Pybus, OG; Markov, PV; Wu, A; Tatem, AJ (Jul 2007). "Investigating the endemic transmission of the hepatitis C virus". Int J Parasitol. 37 (8–9): 839–49. doi:10.1016/j.ijpara.2007.04.009. PMID 17521655.
- ↑ McHenry Harris; Randall E. Harris (2013). Epidemiology of Chronic Disease. Jones & Bartlett Publishers. ISBN 978-0-7637-8047-0. Archived from the original on 2023-09-04. Retrieved 2023-09-03.
- ↑ Rauch, A.; Gaudieri, S.; Thio, C.; Bochud, P. Y. (2009). "Host genetic determinants of spontaneous hepatitis C clearance". Pharmacogenomics. 10 (11): 1819–1837. doi:10.2217/pgs.09.121. PMID 19891557.
- ↑ Meier V, Ramadori G (April 2009). "Hepatitis C virus virology and new treatment targets". Expert Rev Anti Infect Ther. 7 (3): 329–50. doi:10.1586/eri.09.12. PMID 19344246. S2CID 38411966.
- ↑ Manns MP, Foster GR, Rockstroh JK, Zeuzem S, Zoulim F, Houghton M (December 2007). "The way forward in HCV treatment—finding the right path". Nat Rev Drug Discov. 6 (12): 991–1000. doi:10.1038/nrd2411. PMID 18049473. S2CID 52874660.
- ↑ Ahmed, Ali Mahmoud; Doheim, Mohamed Fahmy; Mattar, Omar Mohamed; Sherif, Nourin Ali; Truong, Duy Hieu; Pham T.L., Hoa; Hirayama, Kenji; Huy, Nguyen Tien (May 2018). "Beclabuvir in combination with asunaprevir and daclatasvir for hepatitis C virus genotype 1 infection: A systematic review and meta-analysis". J Med Virol. 90 (5): 907–918. doi:10.1002/jmv.24947. PMID 28892235. S2CID 3829214.
- ↑ "FDA approves Victrelis for Hepatitis C" (press release). FDA. May 13, 2011. Archived from the original on January 18, 2017. Retrieved January 30, 2023.
- ↑ Gutierrez JA, Parikh N, Branch AD (2011). "Classical and emerging roles of vitamin d in hepatitis C virus infection". Semin Liver Dis. 31 (4): 387–398. doi:10.1055/s-0031-1297927. PMC 4107414. PMID 22189978.
- ↑ Lange CM, Bojunga J, Ramos-Lopez E, von Wagner M, Hassler A, Vermehren J, Herrmann E, Badenhoop K, Zeuzem S, et al. (2011). "Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy". J Hepatol. 54 (5): 887–893. doi:10.1016/j.jhep.2010.08.036. PMID 21145801. Archived from the original on 2 May 2020. Retrieved 10 July 2020.
- ↑ Baur K, Mertens JC, Schmitt J, et al. (2012). "The vitamin D receptor gene bAt (CCA) haplotype impairs the response to pegylated-interferon/ribavirin-based therapy in chronic hepatitis C patients". Antivir. Ther. 17 (3): 541–7. doi:10.3851/IMP2018. PMID 22300961. S2CID 32175340.
- ↑ Bitetto D, Fattovich G, Fabris C, Ceriani E, Falleti E, Fornasiere E, Pasino M, Ieluzzi D, Cussigh A, et al. (2011). "Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C". Hepatology. 53 (4): 1118–26. doi:10.1002/hep.24201. PMID 21480318. S2CID 5329252.
- ↑ Gal-Tanamy M, Bachmetov L, Ravid A, Koren R, Erman A, Tur-Kaspa R, Zemel R (2011). "Vitamin D: an innate antiviral agent suppressing hepatitis C virus in human hepatocytes". Hepatology. 54 (5): 1570–9. doi:10.1002/hep.24575. PMID 21793032. S2CID 10090454.
- ↑ Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N (2011). "Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients". World J Gastroenterol. 17 (47): 5184–90. doi:10.3748/wjg.v17.i47.5184. PMC 3243885. PMID 22215943.
- ↑ Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, Bignulin S, Cmet S, Fontanini E, et al. (2011). "Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C". Transpl Int. 24 (1): 43–50. doi:10.1111/j.1432-2277.2010.01141.x. PMID 20649944. S2CID 22124427.
- ↑ Cholongitas E, Theocharidou E, Goulis J, Tsochatzis E, Akriviadis E, Burroughs K (March 2012). "Review article: the extra-skeletal effects of vitamin D in chronic hepatitis C infection". Aliment. Pharmacol. Ther. 35 (6): 634–46. doi:10.1111/j.1365-2036.2012.05000.x. PMID 22316435. S2CID 25534747.
- ↑ Cacopardo B, Camma C, Petta S, Pinzone MR, Cappellani A, Zanghi A, Nicolosi A, Nunnari G (2012). "Diagnostic and therapeutical role of vitamin D in chronic hepatitis C virus infection". Front Biosci. 1 (4): 1276–1286. doi:10.2741/e458. PMID 22201953.
- 1 2 3 Halegoua-De Marzio, Dina; Fenkel, Jonathan (January 27, 2014). "Alternative medications in Hepatitis C infection". World Journal of Hepatology. 6 (1): 9–16. doi:10.4254/wjh.v6.i1.9. PMC 3953807. PMID 24653790.
- ↑ Sarrazin C, Hézode C, Zeuzem S, Pawlotsky JM (2012). "Antiviral strategies in hepatitis C virus infection". J. Hepatol. 56 (Suppl 1): S88–100. doi:10.1016/S0168-8278(12)60010-5. PMID 22300469.
- ↑ "Press announcement, FDA, December 6 2013". Food and Drug Administration. Archived from the original on 2013-12-09. Retrieved 2023-01-30.
- ↑ "FDA approves new treatment for hepatitis C virus". Food and Drug Administration. Nov 22, 2013. Archived from the original on December 16, 2013. Retrieved January 30, 2023.
- ↑ "Medivir: Simeprevir has been approved in Japan for the treatment of genotype 1 chronic hepatitis C infection". The Wall Street Journal. September 27, 2013.
{{cite web}}: CS1 maint: url-status (link)
External links
| Wikispecies has information related to Hepatitis C virus |
- Academic articles about the HCV six genotypes Archived 2020-10-11 at the Wayback Machine Clodovero Ferri
- HCV Sequence and Immunology Databases Archived 2016-05-01 at the Wayback Machine at Los Alamos National Laboratory
- Virus Pathogen Database and Analysis Resource (ViPR): Flaviviridae Archived 2019-09-19 at the Wayback Machine