Progressive multifocal leukoencephalopathy
| Progressive multifocal leukoencephalopathy | |
|---|---|
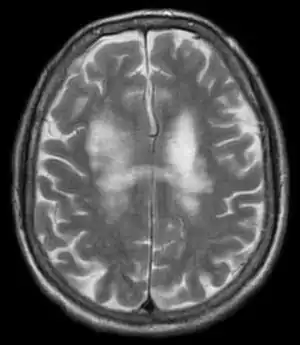 | |
| T2-weighted MRI showing progressive multifocal leukoencephalopathy | |
Progressive multifocal leukoencephalopathy (PML) is a rare and often fatal viral disease characterized by progressive damage (-pathy) or inflammation of the white matter (leuko-) of the brain (-encephalo-) at multiple locations (multifocal). It is caused by the JC virus, which is normally present and kept under control by the immune system. The JC virus is harmless except in cases of weakened immune systems. In general, PML has a mortality rate of 30–50% in the first few months, and those who survive can be left with varying degrees of neurological disabilities.
PML occurs almost exclusively in patients with severe immune deficiency, most commonly among patients with acquired immune deficiency syndrome (AIDS), but people on chronic immunosuppressive medications including chemotherapy are also at increased risk of PML, such as patients with transplants, Hodgkin's lymphoma, multiple sclerosis, psoriasis, rheumatoid arthritis, and other autoimmune diseases.
Signs and symptoms
Symptoms can develop over several weeks to months, and they depend on location of damage in the brain and the degree of damage. The most prominent symptoms are "clumsiness, progressive weakness, and visual, speech, and sometimes personality changes".[1] The lesions affecting the parietal and occipital lobes of the brain can lead to a phenomenon known as alien hand syndrome.[2]
Cause
JC virus infection
The cause of PML is a type of polyomavirus called the JC virus (JCV), after the initials of the person (John Cunningham) from whose tissue the virus was first successfully cultured. Recent publications indicate 39[3] to 58%[4] of the general population are seropositive for antibodies to JCV, indicating current or previous infection with the virus. Other publications put the percentage at 70 to 90% of the general population.[5] JCV causes persistent asymptomatic infection in about one-third of the adult population, based on viral shedding into the urine from the site of asymptomatic infection in the kidney. The virus causes disease only when the immune system has been severely weakened.
Immunosuppression
PML is most common in people with HIV1 infection; prior to the advent of effective antiretroviral therapy, as many as 5% of people with AIDS eventually developed PML.[1] Why PML occurs more frequently in people with AIDS than in other immunosuppressive conditions is unclear; some research suggests the effects of HIV on brain tissue, or on JCV itself, make JCV more likely to become active in the brain and increase its damaging inflammatory effects.[6]
PML can still occur in people on immunosuppressive therapy, such as efalizumab, belatacept, and various transplant drugs, which are meant to weaken the immune system.[7]
Multiple sclerosis medications
Natalizumab (Tysabri) was approved in 2004 by the FDA for MS. It was subsequently withdrawn from the market by its manufacturer after it was linked with three cases of PML.[7] All three initial cases were taking natalizumab in combination with interferon beta-1a.[7] After a safety review, the drug was returned to the market in 2006 as a monotherapy for MS under a special prescription program.[7] As of May 2011, over 130 cases of PML had been reported in MS patients, all in patients who had taken natalizumab for more than a year.[7] While none of them had taken the drug in combination with other disease-modifying treatments, previous use of MS treatments increases the risk of PML between three- and four-fold.[7] The estimated prevalence of PML in MS is 1.5 cases per thousand natalizumab users.[7] Around 20% of MS patients with PML die, and most of the rest are very disabled.[7] One case study describes an MS patient who, during a 4-year course of dimethyl fumarate, developed PML and died.[8]
Fingolimod (Gilenya) was approved in 2010 Archived 2020-10-29 at the Wayback Machine by the FDA for MS. In 2015, the first case of PML and a case of "probable PML" were reported by two Gilenya users that could not be tied to previous immunosuppressant therapies. These new cases are now being added to the drug information sheet included with every prescription (i.e. the "drug label").[9]
Pathogenesis
PML is a demyelinating disease, in which the myelin sheath covering the axons of nerve cells is gradually destroyed, impairing the transmission of nerve impulses. It affects the subcortical white matter, particularly that of the parietal and occipital lobes. PML destroys oligodendrocytes and produces intranuclear inclusions. It is similar to another demyelinating disease, MS, but progresses much more quickly. The breakdown of myelin is proportional to the degree of immunocompromise.[10]
Diagnosis
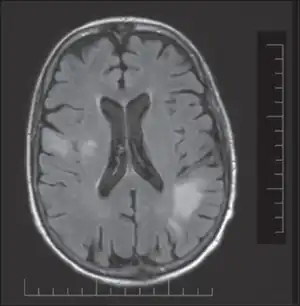
PML is diagnosed in a patient with a progressive course of the disease, finding JC virus DNA in spinal fluid together with consistent white-matter lesions on brain magnetic resonance imaging (MRI); alternatively, a brain biopsy is diagnostic[1] when the typical histopathology of demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei are present, coupled with techniques showing the presence of JC virus.[11]
Characteristic evidence of PML on brain CT scan images are multifocal, noncontrast enhancing hypodense lesions without mass effect, but MRI is far more sensitive than CT.[11] The most common area of involvement is the cortical white matter of frontal and parieto occipital lobes, but lesions may occur anywhere in the brain, such as the basal ganglia, external capsule, and posterior cranial fossa structures such as the brain stem and cerebellum.[11] Although typically multifocal, natalizumab-associated PML is often monofocal, predominantly in the frontal lobe.[11]
Treatment
No drugs effectively inhibit or cure the virus infection without toxicity. Therefore, treatment aims at reversing the immune deficiency to slow or stop the disease progress. In patients on immunosuppression, this means stopping the drugs or using plasma exchange to accelerate the removal of the biologic agent that put the person at risk for PML.[1]
In HIV-infected people, this may mean starting highly active antiretroviral therapy (HAART). AIDS patients starting HAART after being diagnosed with PML tend to have a slightly longer survival time than patients who were already on HAART and then develop PML.[12] Some AIDS patients with PML have been able to survive for several years, with HAART.[13] A rare complication of effective HAART is immune reconstitution inflammatory syndrome (IRIS), in which increased immune system activity actually increases the damage caused by the JCV infection; although IRIS can often be managed with medication, it is extremely dangerous in PML.[14]
Cidofovir was studied as possible treatment for PML[15] and has been used on a case-by-case basis, working in some, but not others.
Cytarabine (also known as ARA-C), a chemotherapy drug used to treat certain cancers, has been prescribed on an experimental basis for a small number of non-AIDS PML patients, and stabilized the neurological condition of a minority of these patients.[16] One patient regained some cognitive function lost as a result of PML.[17]
In June 2010, the first case report appeared of a PML patient being successfully treated with the antimalarial drug mefloquine with activity against the JC virus. The patient cleared the virus and had no further neurological deterioration.[18] Two case reports of using interleukin-2 successfully have been published.[19] Some success have been reported with mirtazapine, but this has not been demonstrated in clinical trials.[20] A number of drugs work against JC virus in cell culture, but no proven, effective therapy is known in humans.[21] For example, 1-O-hexadecyloxypropyl-cidofovir (CMX001), suppresses JCV,[22] but has been found to have toxicity at therapeutic dosage.[23] Infusion of donor T cells specific to the related BK polyomavirus has shown possible effect in treating PML in one small study by Katy Rezvani's group, but needs further study.[24]
Prognosis
One-third to one-half of people with PML die in the first few months following diagnosis, depending on the severity of their underlying disease. Survivors can be left with variable degrees of neurological disability.[1]
See also
- Joseph Berger (neurologist)
- Leukoencephalopathy
- Leukoencephalopathy with vanishing white matter
- Toxic leukoencephalopathy
- Pedro Zamora
- It's_My_Party_(film)
References
- 1 2 3 4 5 "Progressive Multifocal Leukoencephalopathy Information Page". National Institute of Neurological Disorders and Stroke. National Institutes of Health. 14 February 2014. Archived from the original on 17 September 2020. Retrieved 11 September 2020.
- ↑ Panikkath, Ragesh; Panikkath, Deepa; Mojumder, Deb; Nugent, Kenneth (2014). "The alien hand syndrome". Proceedings (Baylor University. Medical Center). 27 (3): 219–220. doi:10.1080/08998280.2014.11929115. PMC 4059570. PMID 24982566.
- ↑ Kean, Jaime M.; Rao, Suchitra; Wang, Michael; Garcea, Robert L. (2009). Atwood, Walter J. (ed.). "Seroepidemiology of Human Polyomaviruses". PLOS Pathogens. 5 (3): e1000363. doi:10.1371/journal.ppat.1000363. PMC 2655709. PMID 19325891.
- ↑ Egli, Adrian; Infanti, Laura; Dumoulin, Alexis; Buser, Andreas; Samaridis, Jacqueline; Stebler, Christine; Gosert, Rainer; Hirsch, Hans H. (2009). "Prevalence of Polyomavirus BK and JC Infection and Replication in 400 Healthy Blood Donors". The Journal of Infectious Diseases. 199 (6): 837–46. doi:10.1086/597126. PMID 19434930.
- ↑ Laura A. Shackelton; Andrew Rambaut; Oliver G. Pybus; Edward C. Holmes (2006). "JC Virus evolution and its association with human populations". Journal of Virology. 80 (20): 9928–9933. doi:10.1128/JVI.00441-06. PMC 1617318. PMID 17005670.
- ↑ Berger, Joseph (2003). "Progressive multifocal leukoencephalopathy in acquired immunodeficiency syndrome: explaining the high incidence and disproportionate frequency of the illness relative to other immunosuppressive conditions". J. Neurovirol. 9 Suppl 1 (2): 38–41. doi:10.1080/13550280390195261. PMID 12709870. S2CID 17171153.
- 1 2 3 4 5 6 7 8 Kappos, Ludwig; Bates, David; Edan, Gilles; Eraksoy, Mefkûre; Garcia-Merino, Antonio; Grigoriadis, Nikolaos; Hartung, Hans-Peter; Havrdová, Eva; Hillert, Jan; Hohlfeld, Reinhard; Kremenchutzky, Marcelo; Lyon-Caen, Olivier; Miller, Ariel; Pozzilli, Carlo; Ravnborg, Mads; Saida, Takahiko; Sindic, Christian; Vass, Karl; Clifford, David B; Hauser, Stephen; Major, Eugene O; O'Connor, Paul W; Weiner, Howard L; Clanet, Michel; Gold, Ralf; Hirsch, Hans H; Radü, Ernst-Wilhelm; Sørensen, Per Soelberg; King, John (August 2011). "Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring". Lancet Neurology. 10 (8): 745–58. doi:10.1016/S1474-4422(11)70149-1. hdl:2078.1/124907. PMID 21777829. S2CID 15639613.
- ↑ "Dimethyl fumarate (Tecfidera): Fatal PML in an MS patient with severe, prolonged lymphopenia". Archived from the original on 2020-11-28. Retrieved 2022-01-26.
- ↑ "FDA Drug Safety Communication: FDA warns about cases of rare brain infection with MS drug Gilenya (fingolimod) in two patients with no prior exposure to immunosuppressant drugs". US Food and Drug Administration. Archived from the original on 8 February 2019. Retrieved 31 December 2018.
- ↑ Radswiki. "Progressive multifocal leukoencephalopathy | Radiology Reference Article | Radiopaedia.org". Radiopaedia. Archived from the original on 2020-07-28. Retrieved 2019-03-09.
- 1 2 3 4 Berger JR; Aksamit AJ; Clifford DB; et al. (9 April 2013). "PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section". Neurology. 80 (15): 1430–8. doi:10.1212/WNL.0b013e31828c2fa1. PMC 3662270. PMID 23568998.
- ↑ Wyen, Christoph; Hoffmann, Christian; Schmeier, Norbert; Hoffmann, Andrej; Qurishi, Nazifa; Rockstroh, Jürgen; Esser, Stefan; Rieke, Ansgar; Ross, Birgit; Lorenzen, Thore; Schmitz, Karina; Stenzel, Werner; Salzberger, Bernd; Fätkenheuer, Gerd (2004). "Progressive multifocal leukencephalopathy in patients on highly active antiretroviral therapy: survival and risk factors of death". Journal of Acquired Immune Deficiency Syndromes. 37 (2): 1263–1268. doi:10.1097/01.qai.0000136093.47316.f3. PMID 15385733. S2CID 23613081.
- ↑ Gray, Françoise; Salmon, Dominique; Thiebault, Jean Baptiste; Gasnault, Jacques; Bienvenu, Boris; Vendrely, Aurélie (2005). "Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy". Acta Neuropathologica. 109 (4): 449–455. doi:10.1007/s00401-005-0983-y. PMID 15739098. S2CID 23865244.
- ↑ Vendrely, Aurélie; Bienvenu, Boris; Gasnault, Jacques; Thiebault, Jean Baptiste; Salmon, Dominique; Gray, Françoise (April 2005). "Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy". Acta Neuropathol. 109 (4): 449–55. doi:10.1007/s00401-005-0983-y. PMID 15739098. S2CID 23865244.
- ↑ Segarra-Newnham, Marisel; Vodolo, Kristen M (June 2001). "Use of cidofovir in progressive multifocal leukoencephalopathy". Ann Pharmacother. 35 (6): 741–4. doi:10.1345/aph.10338. PMID 11408993. S2CID 32026770.
- ↑ Aksamit, A J (August 2001). "Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside". J. Neurovirol. 7 (4): 386–90. doi:10.1080/13550280152537292. PMID 11517422. S2CID 12159275.
- ↑ Langer-Gould, Annette; Atlas, Scott W.; Green, Ari J.; Bollen, Andrew W.; Pelletier, Daniel (July 2005). "Progressive multifocal leukoencephalopathy in a patient treated with natalizumab". N. Engl. J. Med. 353 (4): 375–81. doi:10.1056/NEJMoa051847. PMID 15947078.
- ↑ Gofton, T. E.; Al-Khotani, A.; O'Farrell, B.; Ang, L. C.; McLachlan, R. S. (June 2010). "Mefloquine in the treatment of progressive multifocal leukoencephalopathy". J Neurol Neurosurg Psychiatry. 82 (4): 452–455. doi:10.1136/jnnp.2009.190652. PMID 20562463. S2CID 19877728. Archived from the original on 2012-03-21. Retrieved 2022-01-26.
- ↑ Buckanovich RJ1, Liu G, Stricker C; et al. (July 2002). "Nonmyeloablative allogeneic stem cell transplantation for refractory Hodgkin's lymphoma complicated by interleukin-2 responsive progressive multifocal leukoencephalopathy". Ann Hematol. 81 (7): 410–3. doi:10.1007/s00277-002-0481-4. PMID 12185517. S2CID 24949477.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Jamilloux, Y; Kerever, S; Ferry, T; Broussolle, C; Honnorat, J; Sève, P (October 2016). "Treatment of Progressive Multifocal Leukoencephalopathy With Mirtazapine". Clinical Drug Investigation. 36 (10): 783–9. doi:10.1007/s40261-016-0433-8. PMID 27401779. S2CID 34008609.
- ↑ Ferenczy, MW; Marshall, LJ; Nelson, CD; Atwood, WJ; Nath, A; Khalili, K; Major, EO (July 2012). "Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain". Clin. Microbiol. Rev. 25 (3): 471–506. doi:10.1128/CMR.05031-11. PMC 3416490. PMID 22763635.
- ↑ Gosert, R; Rinaldo, C.H.; Wernil, M; Major, EO; Hirsch, HH (May 2011). "CMX001 (1-O-hexadecyloxypropyl-cidofovir) inhibits polyomavirus JC replication in human brain progenitor-derived astrocytes". Antimicrob. Agents Chemother. 55 (5): 2129–36. doi:10.1128/AAC.00046-11. PMC 3088264. PMID 21402853.
- ↑ Rainer, Gosert; Rinaldo, Christine; Wernli, Marion; Major, Eugene; Hirsch, Hans (2011). "CMX001 (1-O-Hexadecyloxypropyl-Cidofovir) Inhibits Polyomavirus JC Replication in Human Brain Progenitor-Derived Astrocytes". Antimicrobial Agents and Chemotherapy. 55 (5): 2129–2136. doi:10.1128/AAC.00046-11. PMC 3088264. PMID 21402853.
- ↑ Muftuoglu, Muharrem; Olson, Amanda; Marin, David; et al. (2018-10-11). "Allogeneic BK Virus–Specific T Cells for Progressive Multifocal Leukoencephalopathy". New England Journal of Medicine. 379 (15): 1443–1451. doi:10.1056/nejmoa1801540. ISSN 0028-4793. PMC 6283403. PMID 30304652.
External links
- Overview at NIH
- HIV-1 Associated Opportunistic Infections: PML at eMedicine
- MedlinePlus Encyclopedia: Progressive multifocal leukoencephalopathy
| Classification | |
|---|---|
| External resources |
|