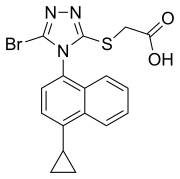
Lesinurad
 | |
 | |
| Names | |
|---|---|
| Pronunciation | Zurampic /zəˈræmpɪk/ zə-RAM-pik |
| Trade names | Zurampic |
IUPAC name
| |
| Clinical data | |
| Main uses | Gout[1] |
| Side effects | Headache, kidney problems, GERD[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth (tablets) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616015 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | ~100%[2] |
| Protein binding | >98% |
| Metabolism | Liver (CYP2C9) |
| Elimination half-life | ~5 hours |
| Excretion | Urine (63%), feces (32%) |
| Chemical and physical data | |
| Formula | C17H14BrN3O2S |
| Molar mass | 404.28 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Lesinurad, sold under the brand name Zurampic, is a medication used to treat high blood uric acid associated with gout.[1] It is only recommended with either allopurinol or febuxostat when these medications are not sufficient.[1] It is taken by mouth.[1]
Common side effects include headache, kidney problems, and GERD.[1] Other side effects may include heart problems and stroke.[1] Safety in pregnancy is unclear.[3] It works by blocking URAT1 and OAT4.[1]
Lesinurad was approved for medical use in the United States in 2015.[1] While it was approved in Europe in 2016, this approval was subsequently withdrawn.[4] It is no longer commercially available in the United States as of 2021.[5]
Medical uses
Lesinurad is used in combination with a xanthine oxidase inhibitor, such as allopurinol or febuxostat, for treating hyperuricemia (high levels of uric acid in the blood serum) associated with gout. It is approved only for people who have not achieved target uric acid levels with a xanthine oxidase inhibitor alone.[2]
Contraindications
The drug is contraindicated in people with tumour lysis syndrome or Lesch–Nyhan syndrome (juvenile gout), as well as severe impairment of kidney function, including kidney transplant and hemodialysis patients.[6][7]
Side effects
In clinical trials, serum creatinine (an important marker for kidney function) was elevated in 4.3 to 7.8% of patients depending on the dose, as compared to 2.3% under placebo. Manifest kidney problems were less frequent under the standard dose than under placebo: Kidney failure occurred in 2.1% of placebo patients, in 1.2% of patients with the therapeutic standard dose, and in 3.5% of patients with the double dose. For kidney stones, the frequencies were 1.7%, 0.6% and 2.5%, respectively.[6][7]
Other common side effects were influenza (5.1% vs. 2.7% under placebo), headache (5.3% vs. 4.1%), and gastroesophageal reflux disease (2.7% vs. 0.8%). Hypersensitivity reactions were rare (<0.1%).[6][7]
Interactions
The substance is a mild inducer of the liver enzyme CYP3A4. Some drugs that are metabolized by this enzyme have been shown to be slightly less effective when combined with lesinurad, examples including simvastatin and warfarin. It might also be a mild inducer of CYP2B6. On the other hand, lesinurad concentrations in the blood are decreased by drugs that induce CYP2C9 and increased by substances that inhibit this enzyme (such as fluconazole), as well as in people who have genetically determined low CYP2C9 activity. The same may be true of microsomal epoxide hydrolase inhibitors (such as valproic acid).[6]
High dose aspirin and related drugs reduce the effectiveness of other anti-gout medications. It is not known conclusively whether this also applies to lesinurad, but low dose aspirin does not negatively affect its activity.[6][7]
Pharmacology
Mechanism of action
Lesinurad inhibits URAT1, a protein that is responsible for reabsorption of uric acid in the kidneys. This leads to increased uric acid excretion with the urine, and consequently lower blood levels. It also inhibits the protein OAT4, which is associated with hyperuricemia caused by diuretic drugs.[6][7]
Pharmacokinetics
Lesinurad is quickly and practically completely absorbed from the gut. Highest blood plasma concentrations are reached after one to four hours. When in the bloodstream, the substance is almost completely (>98%) bound to plasma proteins, mainly albumin.[6][7]
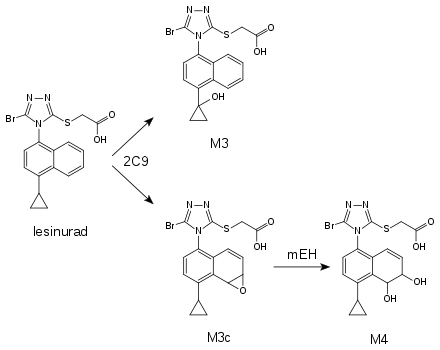
It is metabolized mainly by the liver enzyme CYP2C9 to various oxidation products, predominantly to a hydroxylated substance called M3 and an epoxide, M3c. The latter is quickly hydrolyzed to the diol M4 by microsomal epoxide hydrolase (mEH). The enzymes CYP1A1, CYP2C19 and CYP3A only play minor roles in its metabolization. Glucuronidation by the enzymes UGT1A1 and UGT2B7 has also been detected.[8]
Lesinurad is excreted via the urine (63%) and feces (32%), with a biological half-life of about five hours. Of the excreted dose, 30% are unchanged lesinurad, and the rest are metabolites.[6][7]
Pharmacogenomics
People who are CYP2C9 poor metabolizers are exposed to lesinurad concentrations that are about 1.8-fold higher than those with a normal function of this enzyme.[6][7]
Chemistry
Lesinurad is a white to off-white powder and is not hygroscopic. It is a 1:1 racemic mixture of atropisomers.[9]
See also
- Lesinurad/allopurinol, a fixed-dose combination drug
References
- 1 2 3 4 5 6 7 8 9 "Lesinurad Monograph for Professionals". Drugs.com. Archived from the original on 21 September 2020. Retrieved 21 November 2021.
- 1 2 "Zurampic (lesinurad) Tablets, for Oral Use. Full Prescribing Information" (PDF). AstraZeneca AB, S-151 85 Sodertalje, Sweden. Archived from the original (PDF) on 24 December 2015. Retrieved 23 December 2015.
- ↑ "Lesinurad (Zurampic) Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 21 November 2021.
- ↑ "Zurampic". Archived from the original on 28 August 2021. Retrieved 21 November 2021.
- ↑ "Zurampic Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 1 November 2016. Retrieved 21 November 2021.
- 1 2 3 4 5 6 7 8 9 "Zurampic: EPAR – Product Information" (PDF). European Medicines Agency. 6 July 2017. Archived (PDF) from the original on 18 June 2018. Retrieved 21 July 2021.
- 1 2 3 4 5 6 7 8 FDA Professional Drug Information: Zurampic. Accessed 19 July 2017.
- 1 2 "Zurampic: EPAR – Public assessment report" (PDF). European Medicines Agency. 9 March 2016. pp. 18–19, 38–39. Archived (PDF) from the original on 18 June 2018. Retrieved 21 July 2021.
- ↑ "Zurampic: EPAR – Public assessment report" (PDF). European Medicines Agency. 9 March 2016. p. 9. Archived (PDF) from the original on 18 June 2018. Retrieved 21 July 2021.
External links
- Dean L (2019). "Lesinurad Therapy and CYP2C9 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 30742400. Bookshelf ID: NBK537366. Archived from the original on 26 October 2020. Retrieved 21 July 2021.
| External sites: |
|
|---|---|
| Identifiers: |