Pomalidomide
 | |
| Names | |
|---|---|
| Trade names | Pomalyst, Imnovid |
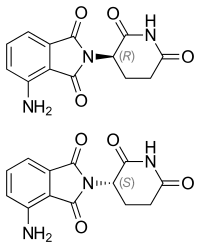
IUPAC name
| |
| Clinical data | |
| Drug class | Thalidomide analog[1] |
| Main uses | Multiple myeloma (MM), Kaposi sarcoma (KS)[2] |
| Side effects | Tiredness, low neutrophils, low red blood cells, nausea, diarrhea, shortness of breath, fever, low platelets[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (capsules) |
| Typical dose | 4 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613030 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 73% (at least)[6] |
| Protein binding | 12–44% |
| Metabolism | Liver (mostly CYP1A2- and CYP3A4-mediated; some minor contributions by CYP2C19 and CYP2D6) |
| Elimination half-life | 7.5 hours |
| Excretion | Urine (73%), faeces (15%) |
| Chemical and physical data | |
| Formula | C13H11N3O4 |
| Molar mass | 273.248 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Pomalidomide, sold under the brand name Pomalyst and Imnovid, is a medication used to treat multiple myeloma (MM) and Kaposi sarcoma (KS).[2] For MM it is used when other treatments have failed.[2] For KS it is used when HAART is not affected or in those who are HIV negative.[2] It is taken by mouth.[2]
Common side effects include tiredness, low neutrophils, low red blood cells, nausea, diarrhea, shortness of breath, fever, and low platelets.[2] Other side effects may include liver problems, tumor lysis syndrome, blood clots, and anaphylaxis.[2] Use in pregnancy may harm the baby.[2] It is similar to thalidomide and works by altering the immune system.[1][5]
Pomalidomide was approved for medical use in the United States and Europe in 2013.[2][5] In the United Kingdom 4 weeks of treatment costs the NHS about £8,900 as of 2021.[7] In the United States this amount costs about 20,000 USD.[8]
Medical uses
Dosage
It is taken at a dose of 4 mg per day for 21 days followed by 7 days off.[1] Lower doses may be used if side effects occur.[1]
Side effects
Pregnancy
Because pomalidomide can cause harm to unborn babies when administered during pregnancy, women taking pomalidomide must not become pregnant.[5]
To avoid embryo-fetal exposure, a "Risk Evaluation and Mitigation Strategy" (REMS) program was developed to ensure pregnancy prevention or distribution of the drug to those who are or might become pregnant.[9] Women must produce two negative pregnancy tests and use contraception methods before beginning pomalidomide. Women must commit either to abstain continuously from heterosexual sexual intercourse or to use two methods of reliable birth control, beginning four weeks prior to initiating treatment with pomalidomide, during therapy, during dose interruptions and continuing for four weeks following discontinuation of pomalidomide therapy.
Pomalidomide is present in the semen of people receiving the drug.[5][2] Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking pomalidomide and for up to 28 days after discontinuing pomalidomide, even if they have undergone a successful vasectomy.[2] Males taking pomalidomide must not donate sperm.[2]
Mechanism of action
Pomalidomide directly inhibits angiogenesis and myeloma cell growth. This dual effect is central to its activity in myeloma, rather than other pathways such as TNF alpha inhibition, since potent TNF inhibitors including rolipram and pentoxifylline do not inhibit myeloma cell growth or angiogenesis.[10] Upregulation of interferon gamma, IL-2 and IL-10 as well as downregulation of IL-6 have been reported for pomalidomide. These changes may contribute to pomalidomide's anti-angiogenic and anti-myeloma activities.
History
The parent compound of pomalidomide, thalidomide, was originally discovered to inhibit angiogenesis in 1994.[11] Based upon this discovery, thalidomide was taken into clinical trials for cancer, leading to its ultimate FDA approval for multiple myeloma.[12] Structure-activity studies revealed that amino substituted thalidomide had improved antitumor activity, which was due to its ability to directly inhibit both the tumor cell and vascular compartments of myeloma cancers.[10] This dual activity of pomalidomide makes it more efficacious than thalidomide in vitro and in vivo.[13]
Research
Phase I trial results showed tolerable side effects.[14]
Phase II clinical trials for multiple myeloma and myelofibrosis reported 'promising results'.[15][16]
Phase III results showed significant extension of progression-free survival, and overall survival (median 11.9 months vs. 7.8 months; p = 0.0002) in patients taking pomalidomide and dexamethasone vs. dexamethasone alone.[17]
See also
- Lenalidomide, another thalidomide analog
- Development of analogs of thalidomide
References
- 1 2 3 4 5 "Pomalidomide Monograph for Professionals". Drugs.com. Archived from the original on 7 September 2015. Retrieved 28 October 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Pomalyst- pomalidomide capsule". DailyMed. 7 December 2017. Archived from the original on 20 October 2020. Retrieved 21 September 2020.
- ↑ "Pomalidomide (Pomalyst) Use During Pregnancy". Drugs.com. 14 May 2020. Archived from the original on 25 January 2021. Retrieved 21 September 2020.
- ↑ "Imnovid 1 mg hard capsules - Summary of Product Characteristics (SmPC)". (emc). 16 June 2020. Archived from the original on 26 October 2020. Retrieved 21 September 2020.
- 1 2 3 4 5 "Imnovid EPAR". European Medicines Agency (EMA). Archived from the original on 27 October 2020. Retrieved 21 September 2020.
- ↑ "Imnovid 1 mg Hard Capsules. Summary of Product Characteristics. 5.2 Pharmacokinetic properties" (PDF). Celgene Europe Ltd. p. 22. Archived (PDF) from the original on 27 June 2016. Retrieved 21 August 2016.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1004. ISBN 978-0857114105.
- ↑ "Pomalyst Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 August 2021. Retrieved 28 October 2021.
- ↑ "Pomalyst Risk Evaluation and Mitigation Strategy (REMS) Program". Celgene Corporation. Archived from the original on 10 August 2016. Retrieved 21 August 2016.
- 1 2 D'Amato RJ, Lentzsch S, Anderson KC, Rogers MS (December 2001). "Mechanism of action of thalidomide and 3-aminothalidomide in multiple myeloma". Seminars in Oncology. 28 (6): 597–601. doi:10.1016/S0093-7754(01)90031-4. PMID 11740816.
- ↑ D'Amato RJ, Loughnan MS, Flynn E, Folkman J (April 1994). "Thalidomide is an inhibitor of angiogenesis". Proceedings of the National Academy of Sciences of the United States of America. 91 (9): 4082–5. Bibcode:1994PNAS...91.4082D. doi:10.1073/pnas.91.9.4082. JSTOR 2364596. PMC 43727. PMID 7513432.
- ↑ Altman, David (2 April 2013). "From Thalidomide to Pomalyst: Better Living Through Chemistry". Archived from the original on 14 May 2021. Retrieved 14 September 2021.
- ↑ Lentzsch S, Rogers MS, LeBlanc R, Birsner AE, Shah JH, Treston AM, et al. (April 2002). "S-3-Amino-phthalimido-glutarimide inhibits angiogenesis and growth of B-cell neoplasias in mice". Cancer Research. 62 (8): 2300–5. PMID 11956087.
- ↑ Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA (April 2008). "Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation". British Journal of Haematology. 141 (1): 41–51. doi:10.1111/j.1365-2141.2008.07013.x. PMID 18324965. S2CID 37073246.
- ↑ "Promising Results From 2 Trials Highlighting Pomalidomide Presented At ASH" (Press release). Celgene. 11 December 2008. Archived from the original on 20 September 2018. Retrieved 28 October 2012.
- ↑ Tefferi, Ayalew (8 December 2008). Pomalidomide Therapy in Anemic Patients with Myelofibrosis: Results from a Phase-2 Randomized Multicenter Study. 50th ASH Annual Meeting and Exposition. San Francisco. Archived from the original on 20 September 2018. Retrieved 28 October 2012.
- ↑ Miguel JS, Weisel K, Moreau P, Lacy M, Song K, Delforge M, et al. (September 2013). "Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial" (PDF). The Lancet. Oncology. 14 (11): 1055–1066. doi:10.1016/s1470-2045(13)70380-2. hdl:2318/150538. PMID 24007748. Archived (PDF) from the original on 20 October 2021. Retrieved 14 September 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |