Acute kidney injury
| Acute kidney injury | |
|---|---|
| Other names: Acute renal failure (ARF)[1] | |
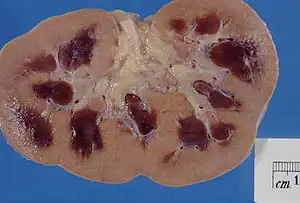 | |
| Kidney specimen showing marked pallor of the cortex, contrasting to the darker areas of surviving medullary tissue in a person with acute kidney injury. | |
| Specialty | Nephrology, urology |
| Types | Prerenal, intrinsic kidney, post-renal[2] |
| Causes | Prerenal: Bleeding, vomiting, diarrhea, sepsis, NSAIDs[2] Intrinsic: acute tubular necrosis, interstitial nephritis, glomerulonephritis[2] Post-renal: kidney stones, bladder cancer, urinary obstruction[2] |
| Diagnostic method | 50% increase serum creatinine in 7 days, 26.5 umol/L (0.3 mg/dL) increase in 2 days, urine output < 0.5 mL/kg per hour x 6hrs[2] |
| Treatment | Prerenal: Intravenous fluids[2] Intrinsic: Supportive care, furosemide, immunosuppressants[2] Post-renal: Urinary tract drainage[2] |
| Prognosis | Often reversible[2] |
| Frequency | Common[2] |
Acute kidney injury (AKI), previously called acute renal failure (ARF), is the sudden decrease in kidney function.[2] Symptoms may include a decrease in urine output.[2] Complications may include pulmonary edema, uremia, and electrolyte abnormalities such as high blood potassium and metabolic acidosis.[2]
Causes can be divided into prerenal, intrinsic kidney, and post-renal.[2] Prerenal causes include anything that decreases blood flow to the kidneys such as bleeding, vomiting, diarrhea, sepsis, and NSAIDs.[2] Intrinsic kidney causes include acute tubular necrosis, interstitial nephritis, and glomerulonephritis.[2] Post-renal causes include kidney stones, bladder cancer, and other causes of urinary obstruction.[2] Diagnosis is based on a 50% increase in serum creatinine within 7 days, a 26.5 umol/L (0.3 mg/dL) within 2 days, or a urine output of less than 0.5 mL/kg per hour for at least six hours.[2]
Management in part depends on the underlying cause.[2] If the bladder is blocked, drainage is required.[2] If a person's blood volume is low intravenous fluid are given.[2] If the kidneys themselves are the reason, furosemide or immunosuppressants may be used.[2] Other supportive measures such as a low potassium diet and dialysis may be required.[2] Often it is reversible.[2]
AKI is common, affecting up to 7% of people admitted to hospital and 30% of people admitted to ICU.[2] In hospitalized people 21% of cases are prerenal, 69% are intrinsic kidney, and 10% are post-renal.[2] Of the intrinsic kidney group acute tubular necrosis is the most common cause.[2] Acute kidney injury was described as early as the Byzantine period (330-1452).[3]
Signs and symptoms
The clinical picture is often dominated by the underlying cause. The various symptoms of acute kidney injury result from the various disturbances of kidney function that are associated with the disease. Accumulation of urea and other nitrogen-containing substances in the bloodstream lead to a number of symptoms, such as fatigue, loss of appetite, headache, nausea and vomiting.[4] Marked increases in the potassium level can lead to abnormal heart rhythms, which can be severe and life-threatening.[5] Fluid balance is frequently affected, though blood pressure can be high, low or normal.[6]
Pain in the flanks may be encountered in some conditions (such as clotting of the kidneys' blood vessels or inflammation of the kidney); this is the result of stretching of the fibrous tissue capsule surrounding the kidney.[7] If the kidney injury is the result of dehydration, there may be thirst as well as evidence of fluid depletion on physical examination.[7] Physical examination may also provide other clues as to the underlying cause of the kidney problem, such as a rash in interstitial nephritis (or vasculitis) and a palpable bladder in obstructive nephropathy.[7]
Causes
AKI can be caused by systemic disease (such as a manifestation of an autoimmune disease, e.g. lupus nephritis), crush injury, contrast agents, some antibiotics, and more. AKI often occurs due to multiple processes. The most common cause is dehydration and sepsis combined with nephrotoxic drugs, especially following surgery or contrast agents.
The causes of acute kidney injury are commonly categorized into prerenal, intrinsic, and postrenal.
In cardiac surgery preoperative creatinine greater than 1.2 mg/dL, combined valve and bypass procedures, emergency surgery, and preoperative intraaortic balloon pump are risk factors most strongly correlated with post-cardiopulmonary bypass acute kidney injury. Other well-known minor risk factors include: female gender, congestive heart failure, chronic obstructive pulmonary disease, insulin-requiring diabetes, and depressed left ventricular ejection fraction.[8]
Prerenal
Prerenal causes of AKI ("pre-renal azotemia") are those that decrease effective blood flow to the kidney and cause a decrease in the glomerular filtration rate (GFR). Both kidneys need to be affected as one kidney is still more than adequate for normal kidney function. Notable causes of prerenal AKI include low blood volume (e.g., dehydration), low blood pressure, heart failure (leading to cardiorenal syndrome), hepatorenal syndrome in the context of liver cirrhosis, and local changes to the blood vessels supplying the kidney. The latter include renal artery stenosis, or the narrowing of the renal artery which supplies the kidney with blood, and renal vein thrombosis, which is the formation of a blood clot in the renal vein that drains blood from the kidney.
Kidneys
Intrinsic AKI refers to disease processes which directly damage the kidney itself. Intrinsic AKI can be due to one or more of the kidney's structures including the glomeruli, kidney tubules, or the interstitium. Common causes of each are glomerulonephritis, acute tubular necrosis (ATN), and acute interstitial nephritis (AIN), respectively. Other causes of intrinsic AKI are rhabdomyolysis and tumor lysis syndrome.[9] Certain medication classes such as calcineurin inhibitors (e.g., tacrolimus) can also directly damage the tubular cells of the kidney and result in a form of intrinsic AKI.
Postrenal
Postrenal AKI refers to acute kidney injury caused by disease states downstream of the kidney and most often occurs as a consequence of urinary tract obstruction. This may be related to benign prostatic hyperplasia, kidney stones, obstructed urinary catheter, bladder stones, or cancer of the bladder, ureters, or prostate.
Diagnosis
| Type | UOsm | UNa | FeNa | BUN/Cr |
|---|---|---|---|---|
| Prerenal | >500 | <10 | <1% | >20[10] |
| Intrinsic | <350 | >20 | >2% | <10-15[10] |
| Postrenal | <350 | >40 | >4% | >20[10] |
Acute kidney injury is diagnosed on the basis of clinical history and laboratory data. A diagnosis is made when there is a rapid reduction in kidney function, as measured by serum creatinine, or based on a rapid reduction in urine output, termed oliguria (less than 400 mLs of urine per 24 hours).
Definition
Introduced by the KDIGO in 2012,[11] specific criteria exist for the diagnosis of AKI.
AKI can be diagnosed if any one of the following is present:
- Increase in SCr by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours; or
- Increase in SCr to ≥1.5 times baseline, which has occurred within the prior 7 days; or
- Urine volume < 0.5 ml/kg/h for 6 hours.
Staging

The RIFLE criteria, proposed by the Acute Dialysis Quality Initiative (ADQI) group, aid in assessment of the severity of a person's acute kidney injury. The acronym RIFLE is used to define the spectrum of progressive kidney injury seen in AKI:[12][13]
- Risk: 1.5-fold increase in the serum creatinine, or glomerular filtration rate (GFR) decrease by 25 percent, or urine output <0.5 mL/kg per hour for six hours.
- Injury: Two-fold increase in the serum creatinine, or GFR decrease by 50 percent, or urine output <0.5 mL/kg per hour for 12 hours
- Failure: Three-fold increase in the serum creatinine, or GFR decrease by 75 percent, or urine output of <0.3 mL/kg per hour for 24 hours, or no urine output (anuria) for 12 hours
- Loss: Complete loss of kidney function (e.g., need for renal replacement therapy) for more than four weeks
- End-stage kidney disease: Complete loss of kidney function (e.g., need for renal replacement therapy) for more than three months
Evaluation
The deterioration of kidney function may be signaled by a measurable decrease in urine output. Often, it is diagnosed on the basis of blood tests for substances normally eliminated by the kidney: urea and creatinine. Additionally, the ratio of BUN to creatinine is used to evaluate kidney injury. Both tests have their disadvantages. For instance, it takes about 24 hours for the creatinine level to rise, even if both kidneys have ceased to function. A number of alternative markers have been proposed (such as NGAL, KIM-1, IL18 and cystatin C), but none of them are currently established enough to replace creatinine as a marker of kidney function.[14]
Once the diagnosis of AKI is made, further testing is often required to determine the underlying cause. It is useful to perform a bladder scan or a post void residual to rule out urinary retention. In post void residual, a catheter is inserted into the urinary tract immediately after urinating to measure fluid still in the bladder. 50–100 ml suggests neurogenic bladder dysfunction.
These may include urine sediment analysis, renal ultrasound and/or kidney biopsy. Indications for kidney biopsy in the setting of AKI include the following:[15]
- Unexplained AKI, in a patient with two non-obstructed normal sized kidneys
- AKI in the presence of the nephritic syndrome
- Systemic disease associated with AKI
- Kidney transplant dysfunction
In medical imaging, the acute changes in the kidney are often examined with renal ultrasonography as the first-line modality, where CT scan and magnetic resonance imaging (MRI) are used for the follow-up examinations and when US fails to demonstrate abnormalities. In evaluation of the acute changes in the kidney, the echogenicity of the renal structures, the delineation of the kidney, the renal vascularity, kidney size and focal abnormalities are observed.[16] CT is preferred in renal traumas, but US is used for follow-up, especially in the patients suspected for the formation of urinomas. A CT scan of the abdomen will also demonstrate bladder distension or hydronephrosis. However, in AKI, the use of IV contrast is contraindicated as the contrast agent used is nephrotoxic.
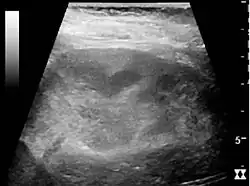 Kidney ultrasonograph of acute pyelonephritis with increased cortical echogenicity and blurred delineation of the upper pole.[16]
Kidney ultrasonograph of acute pyelonephritis with increased cortical echogenicity and blurred delineation of the upper pole.[16] Kidney ultrasonograph in renal failure after surgery with increased cortical echogenicity and kidney size. Biopsy showed acute tubular necrosis.[16]
Kidney ultrasonograph in renal failure after surgery with increased cortical echogenicity and kidney size. Biopsy showed acute tubular necrosis.[16]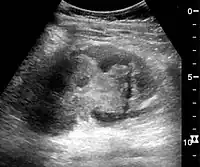 Kidney ultrasonograph in kidney trauma with laceration of the lower pole and subcapsular fluid collection below the kidney.[16]
Kidney ultrasonograph in kidney trauma with laceration of the lower pole and subcapsular fluid collection below the kidney.[16]
Treatment
The management of AKI hinges on identification and treatment of the underlying cause. The main objectives of initial management are to prevent cardiovascular collapse and death and to call for specialist advice from a nephrologist. In addition to treatment of the underlying disorder, management of AKI routinely includes the avoidance of substances that are toxic to the kidneys, called nephrotoxins. These include NSAIDs such as ibuprofen or naproxen, iodinated contrasts such as those used for CT scans, many antibiotics such as gentamicin, and a range of other substances.[17]
Monitoring of kidney function, by serial serum creatinine measurements and monitoring of urine output, is routinely performed. In the hospital, insertion of a urinary catheter helps monitor urine output and relieves possible bladder outlet obstruction, such as with an enlarged prostate.
Prerenal
In prerenal AKI without fluid overload, administration of intravenous fluids is typically the first step to improving kidney function. Volume status may be monitored with the use of a central venous catheter to avoid over- or under-replacement of fluid.
If low blood pressure persists despite providing a person with adequate amounts of intravenous fluid, medications that increase blood pressure (vasopressors) such as norepinephrine and in certain circumstances medications that improve the heart's ability to pump (known as inotropes) such as dobutamine may be given to improve blood flow to the kidney. While a useful vasopressor, there is no evidence to suggest that dopamine is of any specific benefit and may be harmful.[18]
Intrinsic
The myriad causes of intrinsic AKI require specific therapies. For example, intrinsic AKI due to vasculitis or glomerulonephritis may respond to steroid medication, cyclophosphamide, and (in some cases) plasma exchange. Toxin-induced prerenal AKI often responds to discontinuation of the offending agent, such as ACE inhibitors, ARB antagonists, aminoglycosides, penicillins, NSAIDs, or paracetamol.[7]
The use of diuretics such as furosemide, is widespread and sometimes convenient in improving fluid overload. It is not associated with higher mortality (risk of death),[19] nor with any reduced mortality or length of intensive care unit or hospital stay.[20]
Postrenal
If the cause is obstruction of the urinary tract, relief of the obstruction (with a nephrostomy or urinary catheter) may be necessary.
Dialysis
Renal replacement therapy, such as with hemodialysis, may be instituted in some cases of AKI. Renal replacement therapy can be applied intermittently (IRRT) and continuously (CRRT). Study results regarding differences in outcomes between IRRT and CRRT are inconsistent.[21] Among people who are critically ill, intensive renal replacement therapy with continuous venovenous hemofiltration (CVVH) (a type of continuous hemodialysis) does not appear to improve outcomes compared to less intensive intermittent hemodialysis.[17][22] However, other studies demonstrated that compared with IRRT, initiation of CRRT is associated with a lower likelihood of chronic dialysis.[23][24]
In those with AKI without other indications for RRT, starting dialysis within a couple of hours and within a couple of days results in similar outcomes.[25]
Complications
Metabolic acidosis, hyperkalemia, and pulmonary edema may require medical treatment with sodium bicarbonate, antihyperkalemic measures, and diuretics.
Lack of improvement with fluid resuscitation, therapy-resistant hyperkalemia, metabolic acidosis, or fluid overload may necessitate artificial support in the form of dialysis or hemofiltration.[5]
Prognosis
Mortality
Mortality after AKI remains high, AKI has a death rate as high as 20%, which may reach up to 50% in the intensive care unit (ICU). Each year, around two million people die of AKI worldwide.[26]
AKI develops in 5% to 30% of patients who undergo cardiothoracic surgery, depending on the definition used for AKI.[27] If AKI develops after major abdominal surgery (13.4% of all people who have undergone major abdominal surgery) the risk of death is markedly increased (over 12-fold).[28]
Kidney function
Depending on the cause, a proportion of patients (5–10%) will never regain full kidney function, thus entering end-stage kidney failure and requiring lifelong dialysis or a kidney transplant. Patients with AKI are more likely to die prematurely after being discharged from hospital, even if their kidney function has recovered.[29]
The risk of developing chronic kidney disease is increased (8.8-fold).[30]
Epidemiology
New cases of AKI are unusual but not rare, affecting approximately 0.1% of the UK population per year (2000 ppm/year), 20x incidence of new ESKD. AKI requiring dialysis (10% of these) is rare (200 ppm/year), 2x incidence of new ESKD.[31]
There is an increased incidence of AKI in agricultural workers, particularly those paid by the piece. No other traditional risk factors, including age, BMI, diabetes, or hypertension, were associated with incident AKI. Agricultural workers are at increased risk for AKI because of occupational hazards such as dehydration and heat illness.[32]
Acute kidney injury is common among hospitalized patients. It affects some 3–7% of patients admitted to the hospital and approximately 25–30% of patients in the intensive care unit.[33]
Acute kidney injury was one of the most expensive conditions seen in U.S. hospitals in 2011, with an aggregated cost of nearly $4.7 billion for approximately 498,000 hospital stays.[34] This was a 346% increase in hospitalizations from 1997, when there were 98,000 acute kidney injury stays.[35] According to a review article of 2015, there has been an increase in cases of acute kidney injury in the last 20 years which cannot be explained solely by changes to the manner of reporting.[36]
Acute kidney injury occurs in up to 30% of patients following cardiac surgery.[8] Mortality increases by 60-80% in post-cardiopulmonary bypass patients who go on to require renal replacement therapy.
History
Before the advancement of modern medicine, acute kidney injury was referred to as uremic poisoning while uremia was contamination of the blood with urine. Starting around 1847, uremia came to be used for reduced urine output, a condition now called oliguria, which was thought to be caused by the urine's mixing with the blood instead of being voided through the urethra.
Acute kidney injury due to acute tubular necrosis (ATN) was recognized in the 1940s in the United Kingdom, where crush injury victims during the London Blitz developed patchy necrosis of kidney tubules, leading to a sudden decrease in kidney function.[37] During the Korean and Vietnam wars, the incidence of AKI decreased due to better acute management and administration of intravenous fluids.[38]
See also
References
- ↑ Webb S, Dobb G (December 2007). "ARF, ATN or AKI? It's now acute kidney injury". Anaesthesia and Intensive Care. 35 (6): 843–44. doi:10.1177/0310057X0703500601. PMID 18084974.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Goyal, A; Daneshpajouhnejad, P; Hashmi, MF; Bashir, K (January 2020). "Acute Kidney Injury". PMID 28722925.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Congress, International Association for the History of Nephrology (1997). History of Nephrology 2: Reports from the First Congress of the International Association for the History of Nephrology, Kos, Greece, October 14-16, 1996. Karger Medical and Scientific Publishers. p. 217. ISBN 978-3-8055-6499-1. Archived from the original on 2021-08-27. Retrieved 2021-01-27.
- ↑ Skorecki K, Green J, Brenner BM (2005). "Chronic renal failure". In Kasper DL, Braunwald E, Fauci AS, et al. (eds.). Harrison's Principles of Internal Medicine (16th ed.). New York, NY: McGraw-Hill. pp. 1653–63. ISBN 978-0-07-139140-5.
- 1 2 Weisberg LS (December 2008). "Management of severe hyperkalemia". Crit. Care Med. 36 (12): 3246–51. doi:10.1097/CCM.0b013e31818f222b. PMID 18936701. S2CID 33811639.
- ↑ Tierney, Lawrence M.; Stephen J. McPhee; Maxine A. Papadakis (2004). "22". CURRENT Medical Diagnosis and Treatment 2005 (44th ed.). McGraw-Hill. p. 871. ISBN 978-0-07-143692-2.
- 1 2 3 4 Brady HR, Brenner BM (2005). "Chronic renal failure". In Kasper DL, Braunwald E, Fauci AS, et al. (eds.). Harrison's Principles of Internal Medicine (16th ed.). New York, NY: McGraw-Hill. pp. 1644–53. ISBN 978-0-07-139140-5.
- 1 2 Thiele, R. Et al. AKI Associated with Cardiac Surgery. Clinical Journal of the American Society of Nephrology. 2015; Vol 10, n 3.
- ↑ Jim Cassidy; Donald Bissett; Roy A. J. Spence; Miranda Payne (1 January 2010). Oxford Handbook of Oncology. Oxford University Press. p. 706. ISBN 978-0-19-956313-5. Archived from the original on 24 July 2020. Retrieved 3 June 2020.
- 1 2 3 Goldman-Cecil medicine. Goldman, Lee (Physician),, Schafer, Andrew I. (25th ed.). Philadelphia, PA. 15 April 2015. p. 781. ISBN 978-1455750177. OCLC 899727756.
{{cite book}}: CS1 maint: others (link) - ↑ Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter.
- ↑ Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004). "Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group". Crit Care. 8 (4): R204–12. doi:10.1186/cc2872. PMC 522841. PMID 15312219.
- ↑ Lameire N, Van Biesen W, Vanholder R (2005). "Acute renal failure". Lancet. 365 (9457): 417–30. doi:10.1016/S0140-6736(05)17831-3. PMID 15680458.
- ↑ Hall, P (2018). "The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation". Health Technol Assess. 22 (32): 1–274. doi:10.3310/hta22320. PMC 6004543. PMID 29862965.
- ↑ Papadakis MA, McPhee SJ (2008). Current Medical Diagnosis and Treatment. McGraw-Hill Professional. ISBN 978-0-07-159124-9.
- 1 2 3 4 Content initially copied from: Hansen, Kristoffer; Nielsen, Michael; Ewertsen, Caroline (2015). "Ultrasonography of the Kidney: A Pictorial Review". Diagnostics. 6 (1): 2. doi:10.3390/diagnostics6010002. ISSN 2075-4418. PMC 4808817. PMID 26838799. (CC-BY 4.0) Archived 2017-10-16 at the Wayback Machine
- 1 2 Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P (July 2008). "Intensity of renal support in critically ill patients with acute kidney injury". The New England Journal of Medicine. 359 (1): 7–20. doi:10.1056/NEJMoa0802639. PMC 2574780. PMID 18492867.
- ↑ Holmes CL, Walley KR (2003). "Bad medicine: low-dose dopamine in the ICU". Chest. 123 (4): 1266–75. doi:10.1378/chest.123.4.1266. PMID 12684320.
- ↑ Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Nacedo E, Gibney N, Tolwani A, Ronco C, Kellum JA (2004). "Diuretics and mortality in acute renal failure". Crit. Care Med. 32 (8): 1669–77. doi:10.1097/01.CCM.0000132892.51063.2F. PMID 15286542. S2CID 2642777.
- ↑ Davis A, Gooch I (2006). "The use of loop diuretics in acute renal failure in critically ill patients to reduce mortality, maintain renal function, or avoid the requirements for renal support". Emergency Medicine Journal. 23 (7): 569–70. doi:10.1136/emj.2006.038513. PMC 2579558. PMID 16794108.
- ↑ Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M (February 2008). "Renal replacement therapy in patients with acute renal failure: a systematic review". JAMA: The Journal of the American Medical Association. 299 (7): 793–805. doi:10.1001/jama.299.7.793. PMID 18285591.
- ↑ Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S (October 2009). "Intensity of continuous renal-replacement therapy in critically ill patients" (PDF). The New England Journal of Medicine. 361 (17): 1627–38. doi:10.1056/NEJMoa0902413. PMID 19846848. Archived (PDF) from the original on 2021-08-27. Retrieved 2019-05-27.
- ↑ Schoenfelder, T; Chen, X; Bless, HH (March 2017). "Effects of continuous and intermittent renal replacement therapies among adult patients with acute kidney injury". GMS Health Technol Assess. 13 (Doc01 (20170301)). Archived from the original on 8 August 2017. Retrieved 8 August 2017.
- ↑ Schneider, AG; Bellomo, R; Bagshaw, SM; Glassford, NJ; Lo, S; Jun, M; Cass, A; Gallagher, M (June 2013). "Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis" (PDF). Intensive Care Medicine. 39 (6): 987–97. doi:10.1007/s00134-013-2864-5. hdl:11343/218110. PMID 23443311. Archived from the original on 2021-08-27. Retrieved 2019-09-24.
- ↑ Gaudry, Stéphane; Hajage, David; Benichou, Nicolas; Chaïbi, Khalil; Barbar, Saber; Zarbock, Alexander; Lumlertgul, Nuttha; Wald, Ron; Bagshaw, Sean M; Srisawat, Nattachai; Combes, Alain; Geri, Guillaume; Jamale, Tukaram; Dechartres, Agnès; Quenot, Jean-Pierre; Dreyfuss, Didier (May 2020). "Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials". The Lancet. 395 (10235): 1506–1515. doi:10.1016/S0140-6736(20)30531-6.
- ↑ Duan, Shao-Bin; Liu, Fu-You; Liu, Hong; Tang, Mi-Mi; Li, Xu-Wei; Cheng, Wei; Xu, Jun; Yang, Yuan; Luo, Min (2017-08-11). "A new scoring model for the prediction of mortality in patients with acute kidney injury". Scientific Reports. 7 (1): 7862. Bibcode:2017NatSR...7.7862L. doi:10.1038/s41598-017-08440-w. ISSN 2045-2322. PMC 5554175. PMID 28801674.
- ↑ Hobson Charles E.; Yavas Sinan; Segal Mark S.; Schold Jesse D.; Tribble Curtis G.; Layon A. Joseph; Bihorac Azra (2009-05-12). "Acute Kidney Injury Is Associated With Increased Long-Term Mortality After Cardiothoracic Surgery". Circulation. 119 (18): 2444–2453. doi:10.1161/CIRCULATIONAHA.108.800011. PMID 19398670.
- ↑ O'Connor, M. E.; Kirwan, C. J.; Pearse, R. M.; Prowle, J. R. (24 November 2015). "Incidence and associations of acute kidney injury after major abdominal surgery". Intensive Care Medicine. 42 (4): 521–30. doi:10.1007/s00134-015-4157-7. PMID 26602784.
- ↑ Dan Longo; Anthony Fauci; Dennis Kasper; Stephen Hauser; J. Jameson; Joseph Loscalzo (July 21, 2011). Harrison's Principles of Internal Medicine, 18 edition. McGraw-Hill Professional.
- ↑ Coca, SG; Singanamala, S; Parikh, CR (March 2012). "Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis". Kidney International. 81 (5): 442–48. doi:10.1038/ki.2011.379. PMC 3788581. PMID 22113526.
- ↑ "Renal Medicine: Acute Kidney Injury (AKI)". Renalmed.co.uk. 2012-05-23. Archived from the original on 2013-08-08. Retrieved 2013-07-17.
- ↑ Moyce, Sally, RN, BSN; Joseph, Jill, MD, PhD; Tancredi, Daniel, PhD; Mitchell, Diane, PhD; Schenker, Marc, MD, MPH (2016) "Cumulative Incidence of Acute Kidney Injury in California's Agricultural Workers" Journal of Environmental Medicine JOEM 58 Number 4, April 2016 391–97
- ↑ Brenner and Rector's The Kidney. Philadelphia: Saunders. 2007. ISBN 978-1-4160-3110-9.
- ↑ Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. HCUP Statistical Brief #160. Agency for Healthcare Research and Quality, Rockville, MD. August 2013. "Statistical Brief #160". Archived from the original on 2017-03-14. Retrieved 2017-05-01.
- ↑ Pfuntner A., Wier L.M., Stocks C. Most Frequent Conditions in U.S. Hospitals, 2011. HCUP Statistical Brief #162. September 2013. Agency for Healthcare Research and Quality, Rockville, MD. "Most Frequent Conditions in U.S. Hospitals, 2011 #162". Archived from the original on 2016-03-04. Retrieved 2016-02-09.
- ↑ Siew ED, Davenport A (2015). "The growth of acute kidney injury: a rising tide or just closer attention to detail?". Kidney International (Review). 87 (1): 46–61. doi:10.1038/ki.2014.293. PMC 4281297. PMID 25229340.
- ↑ Bywaters EG, Beall D (1941). "Crush injuries with impairment of renal function". Br Med J. 1 (4185): 427–32. doi:10.1136/bmj.1.4185.427. PMC 2161734. PMID 20783577.
- ↑ Schrier RW, Wang W, Poole B, Mitra A (2004). "Acute renal failure: definitions, diagnosis, pathogenesis, and therapy". J. Clin. Invest. 114 (1): 5–14. doi:10.1172/JCI22353. PMC 437979. PMID 15232604.
External links
| Classification | |
|---|---|
| External resources |