Chiral switch
The word "chiral switch" was introduced by Agranat and Caner in 1999.[1] Chiral switches are chiral drugs that are already approved as racemates but that have been re-developed as single enantiomers.[2][3] The term chiral switching has been coined to describe the development of single enantiomers from racemate drugs. For example, levofloxacin is a chiral switch of racemic ofloxacin. The essential principle of a chiral switch is that there is a change in the status of chirality.[4] In general, the term chiral switch is preferred over racemic switch because the switch is usually happening from a racemic drug to the corresponding single enantiomer(s). It is important to understand that chiral switches are treated as a selection invention.[5] A selection invention is an invention that selects a group of new members from a previously known class on the basis of superior properties.[6] To express the pharmacological activities of each of the chiral twins of a racemic drug two technical terms have been coined eutomer and distomer.[7][8] The member of the chiral twin that has greater physiological activity is referred to as the eutomer and the other one with lesser activity is referred to as distomer. The eutomer/distomer ratio is called the eudisimic ratio and reflects the degree of enantioselectivity of the biological activity.[9]
In case of stereoselectivity in action only one of the components in the racemic mixture is truly active (eutomer). The other isomer, the distomer, should be regarded as impurity or isomeric ballast[10] not contributing to the effects aimed at. It is well documented that the pharmacologically inactive isomer (distomer) may contribute to the toxic or adverse effects of the drugs. There is a wide spectrum of possibilities of distomer actions, many of which are confirmed experimentally.[11][12] Sometimes the single enantiomer version lacks certain side-effects that the racemate exhibits. And where the two enantiomers are sufficiently different in pharmacological effects, it may be possible to get a patent on one or both isomers (for instance, as in the case of propoxyphene). The chiral twins of propoxyphene are separately sold by Eli Lilly and company. Dextropropoxyphene is an analgesic agent (Darvon) and levopropoxyphene an effective antitussive (Novrad).[13][14] Interestingly the reversed trade names of the drugs, DARVON and NOVRAD, also reflect the chemical mirror-image relationship. A positive consequence of this redesigning approach is that it has given a new life to an old drug, minimizing or avoiding the undesirable side-effect profile. Whether to go in for a chiral switch is normally made on a case-by-case basis. A pragmatic solution could be in favor of a decision-tree approach, incorporating various factors such as pharmacodynamic, pharmacokinetic, toxicological profile of the enantiomers, enantiomer-enantiomer interaction potential, safety, efficacy, risk-benefit ratio, chiral inversion, distomer liability, physicochemical properties, cost of separation and production, quality control criteria, marketing edge, etc.[15][16][17][18]
The chiral-switch concept
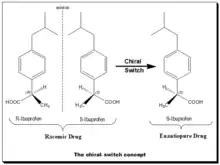
The chiral switch concept[19] is illustrated in the diagram. This chiral switch is from (±)-ibuprofen to (S)-(+)-ibuprofen (dexibuprofen). The nonsteroidal anti-inflammatory drug (NSAID) ibuprofen was the first chiral drug of the NSAID class to be switched to the single-enantiomer version in 1994. The switch was done based on the fact that the (S)-ibuprofen, the eutomer, was over 100-fold more potent as an inhibitor of cycloxygenase-1 (COX-1) enzyme than (R)-ibuprofen.[20] Moreover, ibuprofen, when administered as the racemate, the active (R)-enantiomer undergoes partial unidirectional chiral inversion (approximately 60%) to the (S)-enantiomer. Therefore, the use of the single (S)-ibuprofen was expected to give faster onset of action at a lower dosage.[21]
Rationale for switching
There are several possible potential benefits to chiral switching or chiral specific drugs.[22] These include:
- An improved (less complex, more selective) pharmacodynamic profile
- A higher therapeutic index (improved safety margin)
- Less complex pharmacokinetic profile, less complex drug interactions
- Less complex relationship between plasma concentration and effect
- More rational therapeutic drug monitoring
- Expose the patient to less body load and thus reduce metabolic/renal/hepatic drug load
The chiral switching approach has sometimes resulted in failures and disappointments.[23]
Regulatory environment
The roles of regulatory agencies also continue to evolve with respect to the development of chiral switches. An interesting concept brought up in the FDA policy is that of "bridging studies".[24][25] [26][27] When a sponsor/innovator seeks to develop a single enantiomer from a racemic drug, the regulatory agencies demand them to conduct bridging studies. Bridging studies are tests (pharmacological and toxicological evaluations) to connect what is known about the already approved racemate and what is unknown about the single enantiomer under study, without going back to square one as for a completely new chemical entity. The intent of the bridging studies is to make sure that the companies are not scarifying some protective effect conferred by the other" isomer when they develop a chiral drug as single enantiomer rather than a racemate. "Bridging" procedure will help to reduce the number of studies required on the "new" enantiopure drug.[28]
Launched chiral switches
Chiral switch, a re-engineering approach, has enabled in the remarketing of a number of racemic drugs as chiral specific enantiomer products. Chiral switching strategy is the way most blockbuster drugs have entered the market as enantiopure drugs. But the alternate route is de novo (anew) synthesis of chiral specific drugs.[29] The chiral switches may have the same, very similar, therapeutic indications as the original racemic drug. But, there are instances where new indications for the old drug have been reported. The table below gives a brief list of launched chiral switches.[30]
| Racemic drug | Chiral switch (Unichiral drugs)[31][32] | Pharmacological action | Main benefit(s) claimed |
|---|---|---|---|
| Ibuprofen | (S)-(+)-Ibuprofen; Dexibuprofen | Anti-inflammatory | Faster onset; low adverse effect profile |
| Ofloxacin | (S)-(-)-Ofloxacin; Levofloxacin | Antibactereial | increased potency |
| Ketoprofen | (S)-(+)-Ketoprofen; Dexketoprofen | Anti-inflammatory | Faster onset |
| Salbutamol/ Albuterol | (R)-(-)-Albuterol; Levalbuterol | Bronchodialator | Reduction in side effects; improved tolerability profile |
| Omeprazole | (S)-(-)-Omepazole; Esomeprazole | Proton pump inhibitor | Increased activation; less variable metabolism |
| Bupivacaine | (S)-(-)-Bupivacaine; Levobupivacine | Local anethetic | Decreased risk of cardiotoxicity |
| Cetrizine | (R )-(-)-Cetrizine; Levocetirizine | Antihistamine | Increased potency; decreased side-effects |
| Citalopram | (S)-(-)-Citalopram; Escitalopram | Antidepressant | Faster onset of action; reduction in side effects and improved tolerability profile |
| Ketamine | (S)-Ketamine | Anaesthetic | Increased potency and tolerance; faster recovery |
Failed/aborted chiral switches
The re-evaluation of single enantiomers not without problems. The chiral switches of fluoxetine and fenfluramine are classical examples.[33] The development of (R )-fluoxetine was terminated after patients developed abnormal heart rhythms. The chiral switch of fenfluramine, dexfenfluramine was withdrawn from world marker due to pulmonary hypertension. The table below enumerates couple of chiral switches aborted or withdrawn due stereochemically engineered toxicity.
| Racemic drug | Chiral switch | Pharmacological action | Comments |
| Fluoxetine | (R)-Fluoxetine | Antidepressant | Significant increase in QTC ; Abnormal heart rhythms; Aborted the program[34] |
| Fenfluramine | (S)-Fenfluramine; Dexphenfluramine | Antiobesity | Valvular heart disease and Pulmonary hypertension; withdrawn worldwide,1997.[35][36] |
| Labetalol | Dilevalol | Beta blocker | Increased hepatotoxicity[37] |
| Propranolol | S(-)-Propranolol | Beta blocker | Unexpected reduction of beta-blocking activity[37] |
Evergreening
"Evergreening" refers to the various strategies whereby owners (innovators/sponsors) of pharmaceutical products use patent laws and minor drug modifications to extend their monopoly privileges on the drug.[38] An enantiomer patent is another form of evergreening based on a chiral switch strategy.[39] Single-enantiomer drugs represent more than 50% of the top-selling 100 drugs worldwide.[40] There are some studies which go to suggest that drug companies employ chiral switching for life-cycle management/patent protection of the parent racemic drug and also as a marketing strategy.[41] Pharmaceutical companies support evergreening practices.[42] Some chiral switches are performed to re-start the patent clock for a medication without reducing side effects or improving efficacy.[43] A high price can then continue to be charged for a medication. [43] Examples include citalopram and escitalopram, and omeprazole and esomeprazole. In both these medications, proposed theoretical benefits were used to market the enantiopure drugs, without any clinical trials being conducted to provide evidence that the racemic drugs improved patient centered outcomes. [43]
Metabolite switches
This idea, drug to metabolite switching, is an extension of the chiral switch concept. The purpose of the switching is to develop an active metabolite which will be devoid of the side-effects and have an improved therapeutic profile compared to the parent drug. Some examples of metabolite switches,[44] (including those in the market and others under investigation) include terfenadine to fexofenadine; astemizole to norastemizole; loratadine to desmethylloratadine, halofantrine to desbutylhalofantrine, and cisapride to norcisapride. The following table lists some examples of drug to metabolite switches.
| Original drug | Metabolite switch | Pharmacological action | Main claimed benefit(s) |
|---|---|---|---|
| Terfenadine | Fexofenadine | Antihistaminic | Decreased cardiotoxicity |
| Astemizole | Norastemisole | Antihistaminic | Increased potency; decreased cardiotoxicity |
| Loratidine | Demethyltoratidine | Antihistaminic | Increased potency |
| Halofantrine | Desbutythalofantrine | Antimalarial | Decreased cardiotoxicity |
| Cisapride | Norcisapride | Prokinetic | Increased efficacy; decreased cardiotoxicity |
| Acetanilide | Paracetamol | Analgesic | Does not cause methemoglobinemia |
| Phenacetin | Not carcinogenic | ||
| Risperidone | Paliperidone | Antipsychotic | Less variability of effect; less sedation[45] |
See also
- Chiral drugs
- Chirality
- Enantiopure drug
References
- ↑ Agranat, Israel; Caner, Hava (1999). "Intellectual property and chirality of drugs". Drug Discovery Today. 4 (7): 313–321. doi:10.1016/s1359-6446(99)01363-x. ISSN 1359-6446. PMID 10377509.
- ↑ Agranat, Israel; Wainschtein, Silvya R. (2010). "The strategy of enantiomer patents of drugs". Drug Discovery Today. 15 (5–6): 163–170. doi:10.1016/j.drudis.2010.01.007. ISSN 1359-6446. PMID 20116449.
- ↑ Caner, Hava; Groner, Efrat; Levy, Liron; Agranat, Israel (2004). "Trends in the development of chiral drugs". Drug Discovery Today. 9 (3): 105–110. doi:10.1016/s1359-6446(03)02904-0. ISSN 1359-6446. PMID 15038394.
- ↑ Agranat, Israel; Caner, Hava; Caldwell, John (2002). "Putting chirality to work: the strategy of chiral switches". Nature Reviews Drug Discovery. 1 (10): 753–768. doi:10.1038/nrd915. ISSN 1474-1776. PMID 12360254. S2CID 1543301.
- ↑ Agranat, Israel; Caner, Hava (1999). "Intellectual property and chirality of drugs". Drug Discovery Today. 4 (7): 313–321. doi:10.1016/s1359-6446(99)01363-x. ISSN 1359-6446. PMID 10377509.
- ↑ Grubb, Philip W.; Thomsen, Peter R.; Hoxie, Tom; Wright, Gordon (2016-12-22), "Obtaining a Granted Patent", Patents for Chemicals, Pharmaceuticals, and Biotechnology, Oxford University Press, doi:10.1093/oso/9780199684731.003.0009, ISBN 978-0-19-968473-1, retrieved 2021-05-17
- ↑ Ariens, E. J. (1984). "Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology". European Journal of Clinical Pharmacology. 26 (6): 663–668. doi:10.1007/bf00541922. ISSN 0031-6970. PMID 6092093. S2CID 30916093.
- ↑ Ariëns, Everardus J. (1986). "Stereochemistry: A source of problems in medicinal chemistry". Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404. ISSN 0198-6325. PMID 3534485. S2CID 36115871.
- ↑ Ariëns, Everhardus J.; Wuis, Eveline W.; Veringa, Eric J. (1988). "Stereoselectivity of bioactive xenobiotics". Biochemical Pharmacology. 37 (1): 9–18. doi:10.1016/0006-2952(88)90749-6. ISSN 0006-2952. PMID 3276322.
- ↑ Ariëns, E. J. (1991). "Racemic therapeutics — ethical and regulatory aspects". European Journal of Clinical Pharmacology. 41 (2): 89–93. doi:10.1007/BF00265897. PMID 1743252. S2CID 12768116.
- ↑ Jamali, F.; Mehvar, R.; Pasutto, F.M. (1989). "Enantioselective Aspects of Drug Action and Disposition: Therapeutic Pitfalls". Journal of Pharmaceutical Sciences. 78 (9): 695–715. doi:10.1002/jps.2600780902. ISSN 0022-3549. PMID 2685226.
- ↑ Wright, Matthew R.; Jamali, Fakhreddin (1993). "Methods for the analysis of enantiomers of racemic drugs application to pharmacological and pharmacokinetic studies". Journal of Pharmacological and Toxicological Methods. 29 (1): 1–9. doi:10.1016/1056-8719(93)90044-f. ISSN 1056-8719. PMID 8481555.
- ↑ Drayer, Dennis E (1986). "Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview". Clinical Pharmacology and Therapeutics. 40 (2): 125–133. doi:10.1038/clpt.1986.150. ISSN 0009-9236. PMID 3731675. S2CID 33537650.
- ↑ Ariens, E.J. (1989). Krstulovic, A.M. (ed.). Chiral Separations by HPLC. Ellis Horwwod, Chichester. pp. 31–68.
- ↑ Cayen, Mitchell N. (1991). "Racemic mixtures and single stereoisomers: Industrial concerns and issues in drug development". Chirality. 3 (2): 94–98. doi:10.1002/chir.530030203. ISSN 0899-0042.
- ↑ Evans, AM; Nation, RL; Sansom, LN; Bochner, F.; Somogyi, AA (1988). "Stereoselective drug disposition: potential for misinterpretation of drug disposition data". British Journal of Clinical Pharmacology. 26 (6): 771–780. doi:10.1111/j.1365-2125.1988.tb05318.x. ISSN 0306-5251. PMC 1386594. PMID 3242583.
- ↑ Walle, Thomas; Walle, U.Kristina (1986). "Pharmacokinetic parameters obtained with racemates". Trends in Pharmacological Sciences. 7: 155–158. doi:10.1016/0165-6147(86)90294-4. ISSN 0165-6147.
- ↑ Gross, Michael; Cartwright, Anthony; Campbell, Bruce; Bolton, Roger; Holmes, Keith; Kirkland, Karin; Salmonson, Tomas; Robert, Jean-Louis (1993). "Regulatory Requirements for Chiral Drugs". Drug Information Journal. 27 (2): 453–457. doi:10.1177/009286159302700232. ISSN 0092-8615. S2CID 72629140.
- ↑ Agranat, Israel; Caner, Hava; Caldwell, John (2002). "Putting chirality to work: the strategy of chiral switches". Nature Reviews Drug Discovery. 1 (10): 753–768. doi:10.1038/nrd915. ISSN 1474-1776. PMID 12360254. S2CID 1543301.
- ↑ Mayer, J.M.; Testa, B. (1997). "Pharmacodynamics, pharmacokinetics and toxicity of ibuprofen enantiomers". Drugs of the Future. 22 (12): 1347. doi:10.1358/dof.1997.022.12.711853. ISSN 0377-8282.
- ↑ Caldwell, John; Hutt, Andrew J.; Fournel-Gigleux, Sylvie (1988). "The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences". Biochemical Pharmacology. 37 (1): 105–114. doi:10.1016/0006-2952(88)90762-9. ISSN 0006-2952. PMID 3276314.
- ↑ Tucker, Geoffrey T (2000). "Chiral switches". The Lancet. 355 (9209): 1085–1087. doi:10.1016/s0140-6736(00)02047-x. ISSN 0140-6736. PMID 10744105. S2CID 30715334.
- ↑ Mansfield, Peter; Henry, David; Tonkin, Anne (2004). "Single-Enantiomer Drugs". Clinical Pharmacokinetics. 43 (5): 287–290. doi:10.2165/00003088-200443050-00002. ISSN 0312-5963. PMID 15080762. S2CID 31664339.
- ↑ Tomaszewski, John; Rumore, Martha M. (1994). "Stereoisomeric Drugs: FDA'S Policy Statement and the Impact on Drug Development". Drug Development and Industrial Pharmacy. 20 (2): 119–139. doi:10.3109/03639049409039080. ISSN 0363-9045.
- ↑ Michel, Gross (1991). "Development of chiral drug in an evolving regulatory environment". Regulatory Affairs. 3: 483–494.
- ↑ STINSON, STEPHEN C. (1993-09-27). "CHIRAL DRUGS". Chemical & Engineering News Archive. 71 (39): 38–65. doi:10.1021/cen-v071n039.p038. ISSN 0009-2347.
- ↑ STINSON, STEPHEN C. (1995-10-09). "CHIRAL DRUGS". Chemical & Engineering News Archive. 73 (41): 44–546274. doi:10.1021/cen-v073n041.p044. ISSN 0009-2347.
- ↑ Kumkumian, Charles S. (1990). "Regulatory Considerations concerning Stereoisomers in Drug Products". Drug Information Journal. 24 (1): 125–127. doi:10.1177/009286159002400124. ISSN 0092-8615. S2CID 72604547.
- ↑ Calcaterra, Andrea; D'Acquarica, Ilaria (2018). "The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds". Journal of Pharmaceutical and Biomedical Analysis. 147: 323–340. doi:10.1016/j.jpba.2017.07.008. PMID 28942107. S2CID 6922311.
- ↑ Tucker, Geoffrey T (2000). "Chiral switches". The Lancet. 355 (9209): 1085–1087. doi:10.1016/s0140-6736(00)02047-x. ISSN 0140-6736. PMID 10744105. S2CID 30715334.
- ↑ Gal, Joseph (2006). "Chiral drugs from a historical point of view". In Francotte, Eric; Lindner, Wolfgang (eds.). Chirality in drug research. Germany: Wiley-VCH Verlag GmbH & Co. Germany. pp. 3–26. ISBN 3-527-31076-2.
- ↑ Gal, J (1998). "On the meaning and use of homochiral". Journal of Chromatography A. 829 (1–2): 417–418. doi:10.1016/s0021-9673(98)00845-0. ISSN 0021-9673.
- ↑ Agranat, Israel; Caner, Hava; Caldwell, John (2002). "Putting chirality to work: the strategy of chiral switches". Nature Reviews Drug Discovery. 1 (10): 753–768. doi:10.1038/nrd915. ISSN 1474-1776. PMID 12360254. S2CID 1543301.
- ↑ THAYER, ANN (2000-10-30). "Eli Lilly Pulls The Plug On Prozac Isomer Drug". Chemical & Engineering News Archive. 78 (44): 8. doi:10.1021/cen-v078n044.p008. ISSN 0009-2347.
- ↑ "Valvular Heart Disease Associated with Fenfluramine–Phentermine". New England Journal of Medicine. 337 (24): 1772–1776. 1997-12-11. doi:10.1056/nejm199712113372414. ISSN 0028-4793. PMID 9411246.
- ↑ Anonymous (1997). "Fenfluramine and dexfenfluramine withdrawn. Further cases of valvular heart disease". Current Problems in Pharmacovigilance. 23: 13–14.
- 1 2 https://purehost.bath.ac.uk/ws/portalfiles/portal/278558/Kasprzyk-Hordern_Chem_Soc_Rev_2010_39_11_4466.pdf
- ↑ Alkhafaji, Ali A; Trinquart, Ludovic; Baron, Gabriel; Desvarieux, Moïse; Ravaud, Philippe (2012-11-20). "Impact of evergreening on patients and health insurance: a meta analysis and reimbursement cost analysis of citalopram/escitalopram antidepressants". BMC Medicine. 10 (1): 142. doi:10.1186/1741-7015-10-142. ISSN 1741-7015. PMC 3520785. PMID 23167972.
 Text was copied from this source, which is available under a Creative Commons Attribution 2.0 Generic (CC BY 2.0) license.
Text was copied from this source, which is available under a Creative Commons Attribution 2.0 Generic (CC BY 2.0) license. - ↑ Agranat, Israel; Wainschtein, Silvya R. (2010). "The strategy of enantiomer patents of drugs". Drug Discovery Today. 15 (5–6): 163–170. doi:10.1016/j.drudis.2010.01.007. ISSN 1359-6446. PMID 20116449.
- ↑ Svensson, Staffan; Mansfield, Peter R. (2003-12-12). "Escitalopram: Superior to Citalopram or a Chiral Chimera?". Psychotherapy and Psychosomatics. 73 (1): 10–16. doi:10.1159/000074435. ISSN 0033-3190. PMID 14665791. S2CID 2777719.
- ↑ Mansfield, Peter; Henry, David; Tonkin, Anne (2004). "Single-Enantiomer Drugs". Clinical Pharmacokinetics. 43 (5): 287–290. doi:10.2165/00003088-200443050-00002. ISSN 0312-5963. PMID 15080762. S2CID 31664339.
- ↑ Gaudry, Kate S (2011). "Evergreening: a common practice to protect new drugs". Nature Biotechnology. 29 (10): 876–878. doi:10.1038/nbt.1993. ISSN 1087-0156. PMID 21997625. S2CID 19402161.
- 1 2 3 Somogyi, Andrew; Bochner, Felix; Foster, David (2004). "Inside the isomers: the tale of chiral switches". Australian Prescriber. 27 (2): 47–49. doi:10.18773/austprescr.2004.039. hdl:2440/39339.
- ↑ Tucker, Geoffrey T (2000). "Chiral switches". The Lancet. 355 (9209): 1085–1087. doi:10.1016/s0140-6736(00)02047-x. ISSN 0140-6736. PMID 10744105. S2CID 30715334.
- ↑ https://clinicaltrials.gov/ct2/show/NCT01284959