Metabolic syndrome
| Metabolic syndrome | |
|---|---|
| Other names: Dysmetabolic syndrome X | |
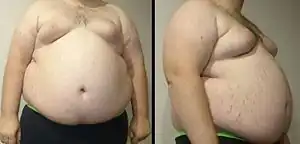 | |
| A man with marked central obesity, a hallmark of metabolic syndrome. His weight is 182 kg (400 lbs), height 185 cm (6 ft 1 in), and body mass index (BMI) 53 (normal 18.5 to 25). | |
| Specialty | Endocrinology |
| Symptoms | Obesity |
| Differential diagnosis | Insulin resistance, prediabetes, hyperuricemia, obesity, nonalcoholic fatty liver disease, polycystic ovarian syndrome, erectile dysfunction, acanthosis nigricans |
| Part of a series on |
| Human body weight |
|---|
Metabolic syndrome is a clustering of at least three of the following five medical conditions: abdominal obesity, high blood pressure, high blood sugar, high serum triglycerides, and low serum high-density lipoprotein (HDL).
Metabolic syndrome is associated with the risk of developing cardiovascular disease and type 2 diabetes.[1] In the U.S., about 25% of the adult population has metabolic syndrome, a proportion increasing with age, particularly among racial and ethnic minorities.[2][3]
Insulin resistance, metabolic syndrome, and prediabetes are closely related to one another and have overlapping aspects. The syndrome is thought to be caused by an underlying disorder of energy utilization and storage. The cause of the syndrome is an area of ongoing medical research.
Signs and symptoms
The key sign of metabolic syndrome is central obesity, also known as visceral, male-pattern or apple-shaped adiposity. It is characterized by adipose tissue accumulation predominantly around the waist and trunk.[4] Other signs of metabolic syndrome include high blood pressure, decreased fasting serum HDL cholesterol, elevated fasting serum triglyceride level, impaired fasting glucose, insulin resistance, or prediabetes. Associated conditions include hyperuricemia; fatty liver (especially in concurrent obesity) progressing to nonalcoholic fatty liver disease; polycystic ovarian syndrome in women and erectile dysfunction in men; and acanthosis nigricans.
Complication
Metabolic syndrome can lead to several serious and chronic complications, including type-2 diabetes, cardiovascular diseases, stroke, kidney disease and nonalcoholic fatty liver disease.[5][6]
Causes
The mechanisms of the complex pathways of metabolic syndrome are under investigation. The pathophysiology is very complex and has been only partially elucidated. Most people affected by the condition are older, obese, sedentary, and have a degree of insulin resistance. Stress can also be a contributing factor. The most important risk factors are diet (particularly sugar-sweetened beverage consumption),[7] genetics,[8][9][10][11] aging, sedentary behavior[12] or low physical activity,[13][14] disrupted chronobiology/sleep,[15] mood disorders/psychotropic medication use,[16][17] and excessive alcohol use.[18] The pathogenic role played in the syndrome by the excessive expansion of adipose tissue occurring under sustained overeating, and its resulting lipotoxicity was reviewed by Vidal-Puig.[19]
There is debate regarding whether obesity or insulin resistance is the cause of the metabolic syndrome or if they are consequences of a more far-reaching metabolic derangement. Markers of systemic inflammation, including C-reactive protein, are often increased, as are fibrinogen, interleukin 6, tumor necrosis factor-alpha (TNF-α), and others. Some have pointed to a variety of causes, including increased uric acid levels caused by dietary fructose.[20][21][22]
Research shows that Western diet habits are a factor in development of metabolic syndrome, with high consumption of food that is not biochemically suited to humans.[23] Weight gain is associated with metabolic syndrome. Rather than total adiposity, the core clinical component of the syndrome is visceral and/or ectopic fat (i.e., fat in organs not designed for fat storage) whereas the principal metabolic abnormality is insulin resistance.[24] The continuous provision of energy via dietary carbohydrate, lipid, and protein fuels, unmatched by physical activity/energy demand, creates a backlog of the products of mitochondrial oxidation, a process associated with progressive mitochondrial dysfunction and insulin resistance.
Stress
Recent research indicates prolonged chronic stress can contribute to metabolic syndrome by disrupting the hormonal balance of the hypothalamic-pituitary-adrenal axis (HPA-axis).[25] A dysfunctional HPA-axis causes high cortisol levels to circulate, which results in raising glucose and insulin levels, which in turn cause insulin-mediated effects on adipose tissue, ultimately promoting visceral adiposity, insulin resistance, dyslipidemia and hypertension, with direct effects on the bone, causing "low turnover" osteoporosis.[26] HPA-axis dysfunction may explain the reported risk indication of abdominal obesity to cardiovascular disease (CVD), type 2 diabetes and stroke.[27] Psychosocial stress is also linked to heart disease.[28]
Obesity
Central obesity is a key feature of the syndrome, being both a sign and a cause, in that the increasing adiposity often reflected in high waist circumference may both result from and contribute to insulin resistance. However, despite the importance of obesity, affected people who are of normal weight may also be insulin-resistant and have the syndrome.[29]
Sedentary lifestyle
Physical inactivity is a predictor of CVD events and related mortality. Many components of metabolic syndrome are associated with a sedentary lifestyle, including increased adipose tissue (predominantly central); reduced HDL cholesterol; and a trend toward increased triglycerides, blood pressure, and glucose in the genetically susceptible. Compared with individuals who watched television or videos or used their computers for less than one hour daily, those who carried out these behaviors for greater than four hours daily have a twofold increased risk of metabolic syndrome.[29]
Aging
Metabolic syndrome affects 60% of the U.S. population older than age 50. With respect to that demographic, the percentage of women having the syndrome is higher than that of men. The age dependency of the syndrome's prevalence is seen in most populations around the world.[29]
Diabetes mellitus type 2
The metabolic syndrome quintuples the risk of type 2 diabetes mellitus. Type 2 diabetes is considered a complication of metabolic syndrome.[1] In people with impaired glucose tolerance or impaired fasting glucose, presence of metabolic syndrome doubles the risk of developing type 2 diabetes.[30] It is likely that prediabetes and metabolic syndrome denote the same disorder, defining it by the different sets of biological markers.
The presence of metabolic syndrome is associated with a higher prevalence of CVD than found in people with type 2 diabetes or impaired glucose tolerance without the syndrome.[29] Hypoadiponectinemia has been shown to increase insulin resistance[31] and is considered to be a risk factor for developing metabolic syndrome.[32]
Coronary heart disease
The approximate prevalence of the metabolic syndrome in people with coronary artery disease (CAD) is 50%, with a prevalence of 37% in people with premature coronary artery disease (age 45), particularly in women. With appropriate cardiac rehabilitation and changes in lifestyle (e.g., nutrition, physical activity, weight reduction, and, in some cases, drugs), the prevalence of the syndrome can be reduced.[29]
Lipodystrophy
Lipodystrophic disorders in general are associated with metabolic syndrome. Both genetic (e.g., Berardinelli-Seip congenital lipodystrophy, Dunnigan familial partial lipodystrophy) and acquired (e.g., HIV-related lipodystrophy in people treated with highly active antiretroviral therapy) forms of lipodystrophy may give rise to severe insulin resistance and many of metabolic syndrome's components.[29]
Rheumatic diseases
There is research that associates comorbidity with rheumatic diseases. Both psoriasis and psoriatic arthritis have been found to be associated with metabolic syndrome.[33]
Chronic obstructive pulmonary disease
Metabolic syndrome is seen to be a comorbidity in up to 50 percent of those with chronic obstructive pulmonary disease (COPD). It may pre-exist or may be a consequence of the lung pathology of COPD.[34]
Pathophysiology
It is common for there to be a development of visceral fat, after which the adipocytes (fat cells) of the visceral fat increase plasma levels of TNF-α and alter levels of other substances (e.g., adiponectin, resistin, and PAI-1). TNF-α has been shown to cause the production of inflammatory cytokines and also possibly trigger cell signaling by interaction with a TNF-α receptor that may lead to insulin resistance.[35] An experiment with rats fed a diet with 33% sucrose has been proposed as a model for the development of metabolic syndrome. The sucrose first elevated blood levels of triglycerides, which induced visceral fat and ultimately resulted in insulin resistance. The progression from visceral fat to increased TNF-α to insulin resistance has some parallels to human development of metabolic syndrome. The increase in adipose tissue also increases the number of immune cells, which play a role in inflammation. Chronic inflammation contributes to an increased risk of hypertension, atherosclerosis and diabetes.[36]
The involvement of the endocannabinoid system in the development of metabolic syndrome is indisputable.[37][38][39] Endocannabinoid overproduction may induce reward system dysfunction[38] and cause executive dysfunctions (e.g., impaired delay discounting), in turn perpetuating unhealthy behaviors. The brain is crucial in development of metabolic syndrome, modulating peripheral carbohydrate and lipid metabolism.[37][38]
Metabolic syndrome can be induced by overfeeding with sucrose or fructose, particularly concomitantly with high-fat diet.[40] The resulting oversupply of omega-6 fatty acids, particularly arachidonic acid (AA), is an important factor in the pathogenesis of metabolic syndrome. Arachidonic acid (with its precursor – linoleic acid) serves as a substrate to the production of inflammatory mediators known as eicosanoids, whereas the arachidonic acid-containing compound diacylglycerol (DAG) is a precursor to the endocannabinoid 2-arachidonoylglycerol (2-AG) while fatty acid amide hydrolase (FAAH) mediates the metabolism of anandamide into arachidonic acid.[41][39] Anandamide can also be produced from N-acylphosphatidylethanolamine via several pathways.[39] Anandamide and 2-AG can also be hydrolized into arachidonic acid, potentially leading to increased eicosanoid synthesis.[39]
Diagnosis
A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity published a guideline to harmonize the definition of the metabolic syndrome.[42] This definition recognizes that the risk associated with a particular waist measurement will differ in different populations. Whether it is better at this time to set the level at which risk starts to increase or at which there is already substantially increased risk will be up to local decision-making groups. However, for international comparisons and to facilitate the etiology, it is critical that a commonly agreed-upon set of criteria be used worldwide, with agreed-upon cut points for different ethnic groups and sexes. There are many people in the world of mixed ethnicity, and in those cases, pragmatic decisions will have to be made. Therefore, an international criterion of overweight may be more appropriate than ethnic specific criteria of abdominal obesity for an anthropometric component of this syndrome which results from an excess lipid storage in adipose tissue, skeletal muscle and liver.
The previous definitions of the metabolic syndrome by the International Diabetes Federation[43] (IDF) and the revised National Cholesterol Education Program (NCEP) are very similar, and they identify individuals with a given set of symptoms as having metabolic syndrome. There are two differences, however: the IDF definition states that if body mass index (BMI) is greater than 30 kg/m2, central obesity can be assumed, and waist circumference does not need to be measured. However, this potentially excludes any subject without increased waist circumference if BMI is less than 30. Conversely, the NCEP definition indicates that metabolic syndrome can be diagnosed based on other criteria. Also, the IDF uses geography-specific cut points for waist circumference, while NCEP uses only one set of cut points for waist circumference regardless of geography.
IDF
The International Diabetes Federation[43] consensus worldwide definition of metabolic syndrome (2006) is: Central obesity (defined as waist circumference# with ethnicity-specific values) AND any two of the following:
- Raised triglycerides: > 150 mg/dL (1.7 mmol/L), or specific treatment for this lipid abnormality
- Reduced HDL cholesterol: < 40 mg/dL (1.03 mmol/L) in males, < 50 mg/dL (1.29 mmol/L) in females, or specific treatment for this lipid abnormality
- Raised blood pressure (BP): systolic BP > 130 or diastolic BP >85 mm Hg, or treatment of previously diagnosed hypertension
- Raised fasting plasma glucose (FPG): >100 mg/dL (5.6 mmol/L), or previously diagnosed type 2 diabetes
If FPG is >5.6 mmol/L or 100 mg/dL, an oral glucose tolerance test is strongly recommended, but is not necessary to define presence of the syndrome.
# If BMI is >30 kg/m2, central obesity can be assumed and waist circumference does not need to be measured
WHO
The World Health Organization (1999)[44] requires the presence of any one of diabetes mellitus, impaired glucose tolerance, impaired fasting glucose or insulin resistance, AND two of the following:
- Blood pressure ≥ 140/90 mmHg
- Dyslipidemia: triglycerides (TG) ≥ 1.695 mmol/L and HDL cholesterol ≤ 0.9 mmol/L (male), ≤ 1.0 mmol/L (female)
- Central obesity: waist:hip ratio > 0.90 (male); > 0.85 (female), or BMI > 30 kg/m2
- Microalbuminuria: urinary albumin excretion ratio ≥ 20 µg/min or albumin:creatinine ratio ≥ 30 mg/g
EGIR
The European Group for the Study of Insulin Resistance (1999) requires insulin resistance defined as the top 25% of the fasting insulin values among nondiabetic individuals AND two or more of the following:
- Central obesity: waist circumference ≥ 94 cm or 37 inches (male), ≥ 80 cm or 31.5 inches (female)
- Dyslipidemia: TG ≥ 2.0 mmol/L and/or HDL-C < 1.0 mmol/L or treated for dyslipidemia
- Blood pressure ≥ 140/90 mmHg or antihypertensive medication
- Fasting plasma glucose ≥ 6.1 mmol/L
NCEP
The U.S. National Cholesterol Education Program Adult Treatment Panel III (2001) requires at least three of the following:[45]
- Central obesity: waist circumference ≥ 102 cm or 40 inches (male), ≥ 88 cm or 35 inches(female)
- Dyslipidemia: TG ≥ 1.7 mmol/L (150 mg/dl)
- Dyslipidemia: HDL-C < 40 mg/dL (male), < 50 mg/dL (female)
- Blood pressure ≥ 130/85 mmHg (or treated for hypertension)
- Fasting plasma glucose ≥ 6.1 mmol/L (110 mg/dl)
American Heart Association
There is confusion as to whether, in 2004, the American Heart Association and National Heart, Lung, and Blood Institute intended to create another set of guidelines or simply update the National Cholesterol Education Program definition.[46][47]
- Central obesity: waist circumference ≥ 102 cm or 40 inches (male), ≥ 88 cm or 35 inches(female)
- Dyslipidemia: TG ≥ 1.7 mmol/L (150 mg/dL)
- Dyslipidemia: HDL-C < 40 mg/dL (male), < 50 mg/dL (female)
- Blood pressure ≥ 130/85 mmHg (or treated for hypertension)
- Fasting plasma glucose ≥ 5.6 mmol/L (100 mg/dL), or use of medication for hyperglycemia
Other
High-sensitivity C-reactive protein has been developed and used as a marker to predict coronary vascular diseases in metabolic syndrome, and it was recently used as a predictor for nonalcoholic fatty liver disease (steatohepatitis) in correlation with serum markers that indicated lipid and glucose metabolism.[48] Fatty liver disease and steatohepatitis can be considered manifestations of metabolic syndrome, indicative of abnormal energy storage as fat in ectopic distribution. Reproductive disorders (such as polycystic ovary syndrome in women of reproductive age), and erectile dysfunction or decreased total testosterone (low testosterone-binding globulin) in men can be attributed to metabolic syndrome.[49]
Prevention
Various strategies have been proposed to prevent the development of metabolic syndrome. These include increased physical activity (such as walking 30 minutes every day),[50] and a healthy, reduced calorie diet.[51] Many studies support the value of a healthy lifestyle as above. However, one study stated these potentially beneficial measures are effective in only a minority of people, primarily because of a lack of compliance with lifestyle and diet changes.[13] The International Obesity Taskforce states that interventions on a sociopolitical level are required to reduce development of the metabolic syndrome in populations.[52]
The Caerphilly Heart Disease Study followed 2,375 male subjects over 20 years and suggested the daily intake of a pint (~568 mL) of milk or equivalent dairy products more than halved the risk of metabolic syndrome.[53] Some subsequent studies support the authors' findings, while others dispute them.[54] A systematic review of four randomized controlled trials said that, in the short term, a paleolithic nutritional pattern improved three of five measurable components of the metabolic syndrome in participants with at least one of the components.[55]
Management
Medications
Generally, the individual disorders that compose the metabolic syndrome are treated separately.[56] Diuretics and ACE inhibitors may be used to treat hypertension. Various cholesterol medications may be useful if LDL cholesterol, triglycerides, and/or HDL cholesterol is abnormal.
Diet
Dietary carbohydrate restriction reduces blood glucose levels, contributes to weight loss, and reduces the use of several medications that may be prescribed for metabolic syndrome.[57]
Epidemiology
Approximately 20–25 percent of the world's adult population has the cluster of risk factors that is metabolic syndrome.[43] In 2000, approximately 32% of U.S. adults had metabolic syndrome.[58][59] In more recent years that figure has climbed to 34%.[59][60]
In young children, there is no consensus on how to measure metabolic syndrome since age-specific cut points and reference values that would indicate "high risk" have not been well established.[61] A continuous cardiometabolic risk summary score is often used for children instead of a dichotomous measure of metabolic syndrome.[62]
History
In 1921, Joslin first reported the association of diabetes with hypertension and hyperuricemia.[63] In 1923, Kylin reported additional studies on the above triad.[64] In 1947, Vague observed that upper body obesity appeared to predispose to diabetes, atherosclerosis, gout and calculi.[65] In the late 1950s, the term metabolic syndrome was first used In 1967, Avogadro, Crepaldi and coworkers described six moderately obese people with diabetes, hypercholesterolemia, and marked hypertriglyceridemia, all of which improved when the affected people were put on a hypocaloric, low-carbohydrate diet.[66] In 1977, Haller used the term "metabolic syndrome" for associations of obesity, diabetes mellitus, hyperlipoproteinemia, hyperuricemia, and hepatic steatosis when describing the additive effects of risk factors on atherosclerosis.[67] The same year, Singer used the term for associations of obesity, gout, diabetes mellitus, and hypertension with hyperlipoproteinemia.[68] In 1977 and 1978, Gerald B. Phillips developed the concept that risk factors for myocardial infarction concur to form a "constellation of abnormalities" (i.e., glucose intolerance, hyperinsulinemia, hypercholesterolemia, hypertriglyceridemia, and hypertension) associated not only with heart disease, but also with aging, obesity and other clinical states. He suggested there must be an underlying linking factor, the identification of which could lead to the prevention of cardiovascular disease; he hypothesized that this factor was sex hormones.[69][70] In 1988, in his Banting lecture, Gerald M. Reaven proposed insulin resistance as the underlying factor and named the constellation of abnormalities syndrome X. Reaven did not include abdominal obesity, which has also been hypothesized as the underlying factor, as part of the condition.[71]
See also
References
- 1 2 "Metabolic syndrome". Mayo Clinic. Archived from the original on 2 September 2020. Retrieved 10 Sep 2020.
- ↑ Falkner B, Cossrow ND (July 2014). "Prevalence of metabolic syndrome and obesity-associated hypertension in the racial ethnic minorities of the United States". Current Hypertension Reports. 16 (7): 449. doi:10.1007/s11906-014-0449-5. PMC 4083846. PMID 24819559.
- ↑ Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S (August 2013). "Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010". Journal of the American College of Cardiology. 62 (8): 697–703. doi:10.1016/j.jacc.2013.05.064. PMC 3756561. PMID 23810877.
- ↑ "Metabolic Syndrome". Diabetes.co.uk. 15 January 2019. Archived from the original on 12 May 2022. Retrieved 17 May 2022.
- ↑ "Metabolic syndrome - Symptoms and causes". Mayo Clinic. Archived from the original on 2022-03-28. Retrieved 2022-03-31.
- ↑ "Metabolic Syndrome: Risk Factors, Diagnosis, and More". Healthline. 2022-01-28. Archived from the original on 2022-03-31. Retrieved 2022-03-31.
- ↑ Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB (November 2010). "Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis". Diabetes Care. 33 (11): 2477–83. doi:10.2337/dc10-1079. PMC 2963518. PMID 20693348.
- ↑ Pollex RL, Hegele RA (September 2006). "Genetic determinants of the metabolic syndrome". Nature Clinical Practice Cardiovascular Medicine. 3 (9): 482–89. doi:10.1038/ncpcardio0638. PMID 16932765. S2CID 24558150.
- ↑ Poulsen P, Vaag A, Kyvik K, Beck-Nielsen H (May 2001). "Genetic versus environmental aetiology of the metabolic syndrome among male and female twins". Diabetologia. 44 (5): 537–43. doi:10.1007/s001250051659. PMID 11380071. S2CID 26582450.
- ↑ Groop, Leif (2007). "Genetics of the metabolic syndrome". British Journal of Nutrition. 83: S39–S48. doi:10.1017/S0007114500000945. PMID 10889791. S2CID 8974554.
- ↑ Bouchard C (May 1995). "Genetics and the metabolic syndrome". International Journal of Obesity and Related Metabolic Disorders. 19 Suppl 1: S52–59. PMID 7550538.
- ↑ Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, Yates T, Biddle SJ (2012). "Association of sedentary behaviour with metabolic syndrome: a meta-analysis". PLOS ONE. 7 (4): e34916. Bibcode:2012PLoSO...734916E. doi:10.1371/journal.pone.0034916. PMC 3325927. PMID 22514690.
- 1 2 Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C (October 2003). "Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study". Medicine and Science in Sports and Exercise. 35 (10): 1703–09. doi:10.1249/01.MSS.0000089337.73244.9B. PMID 14523308.
- ↑ He D, Xi B, Xue J, Huai P, Zhang M, Li J (June 2014). "Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies". Endocrine. 46 (2): 231–40. doi:10.1007/s12020-013-0110-0. PMID 24287790. S2CID 5271746.
- ↑ Xi B, He D, Zhang M, Xue J, Zhou D (August 2014). "Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis". Sleep Medicine Reviews. 18 (4): 293–97. doi:10.1016/j.smrv.2013.06.001. PMID 23890470.
- ↑ Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, Probst M, Scheewe TW, De Hert M (July 2014). "Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables". Psychological Medicine. 44 (10): 2017–28. doi:10.1017/S0033291713002778. PMID 24262678. S2CID 206253750. Archived from the original on 2016-03-26. Retrieved 2022-05-17.
- ↑ Vancampfort D, Vansteelandt K, Correll CU, Mitchell AJ, De Herdt A, Sienaert P, Probst M, De Hert M (March 2013). "Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators". The American Journal of Psychiatry. 170 (3): 265–74. doi:10.1176/appi.ajp.2012.12050620. PMID 23361837.
- ↑ Sun K, Ren M, Liu D, Wang C, Yang C, Yan L (August 2014). "Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies". Clinical Nutrition. 33 (4): 596–602. doi:10.1016/j.clnu.2013.10.003. PMID 24315622.
- ↑ Vidal-Puig, Antonio (2013). "Adipose tissue expandability, lipotoxicity and the metabolic syndrome". Endocrinologia y Nutricion. 60 Suppl 1: 39–43. doi:10.1016/s1575-0922(13)70026-3. ISSN 1579-2021. PMID 24490226. Archived from the original on 2022-03-07. Retrieved 2022-05-17.
- ↑ Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ (March 2006). "A causal role for uric acid in fructose-induced metabolic syndrome". American Journal of Physiology. Renal Physiology. 290 (3): F625–31. doi:10.1152/ajprenal.00140.2005. PMID 16234313.
- ↑ Hallfrisch J (June 1990). "Metabolic effects of dietary fructose". FASEB Journal. 4 (9): 2652–60. doi:10.1096/fasebj.4.9.2189777. PMID 2189777. S2CID 23659634.
- ↑ Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ (May 1989). "Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch". The American Journal of Clinical Nutrition. 49 (5): 832–39. doi:10.1093/ajcn/49.5.832. PMID 2497634.
- ↑ Bremer AA, Mietus-Snyder M, Lustig RH (March 2012). "Toward a unifying hypothesis of metabolic syndrome". Pediatrics. 129 (3): 557–70. doi:10.1542/peds.2011-2912. PMC 3289531. PMID 22351884.
- ↑ Ali ES, Hua J, Wilson CH, Tallis GA, Zhou FH, Rychkov GY, Barritt GJ (September 2016). "The glucagon-like peptide-1 analogue exendin-4 reverses impaired intracellular Ca(2+) signalling in steatotic hepatocytes". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1863 (9): 2135–46. doi:10.1016/j.bbamcr.2016.05.006. PMID 27178543.
- ↑ Gohil BC, Rosenblum LA, Coplan JD, Kral JG (July 2001). "Hypothalamic-pituitary-adrenal axis function and the metabolic syndrome X of obesity". CNS Spectrums. 6 (7): 581–86, 589. doi:10.1017/s1092852900002121. PMID 15573024. S2CID 22734016.
- ↑ Tsigos C, Chrousos GP (October 2002). "Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress". Journal of Psychosomatic Research. 53 (4): 865–71. doi:10.1016/S0022-3999(02)00429-4. PMID 12377295. Archived from the original on 2022-05-18. Retrieved 2022-05-17.
- ↑ Rosmond R, Björntorp P (February 2000). "The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke". Journal of Internal Medicine. 247 (2): 188–97. doi:10.1046/j.1365-2796.2000.00603.x. PMID 10692081. S2CID 20336259.
- ↑ Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG (November 2002). "Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study". Circulation. 106 (21): 2659–65. doi:10.1161/01.cir.0000038364.26310.bd. PMID 12438290. S2CID 5992769.
- 1 2 3 4 5 6 Fauci, Anthony S. (2008). Harrison's principles of internal medicine. McGraw-Hill Medical. ISBN 978-0-07-147692-8.
- ↑ Goldberg RB, Mather K (September 2012). "Targeting the consequences of the metabolic syndrome in the Diabetes Prevention Program". Arteriosclerosis, Thrombosis, and Vascular Biology. 32 (9): 2077–90. doi:10.1161/ATVBAHA.111.241893. PMC 3901161. PMID 22895669.
- ↑ Lara-Castro C, Fu Y, Chung BH, Garvey WT (June 2007). "Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease". Current Opinion in Lipidology. 18 (3): 263–70. doi:10.1097/MOL.0b013e32814a645f. PMID 17495599. S2CID 20799218.
- ↑ Renaldi O, Pramono B, Sinorita H, Purnomo LB, Asdie RH, Asdie AH (January 2009). "Hypoadiponectinemia: a risk factor for metabolic syndrome". Acta Medica Indonesiana. 41 (1): 20–24. PMID 19258676.
- ↑ Quilon, Augusto; Brent, Lawrence (2010). "The primary care physician's guide to inflammatory arthritis: diagnosis". The Journal of Musculoskeletal Medicine. 27: 223–31. Archived from the original on 2012-03-03. Retrieved 2022-05-17.
- ↑ Chan SM, Selemidis S, Bozinovski S, Vlahos R (June 2019). "Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies". Pharmacol Ther. 198: 160–188. doi:10.1016/j.pharmthera.2019.02.013. PMC 7112632. PMID 30822464.
- ↑ Hotamisligil GS (June 1999). "The role of TNFalpha and TNF receptors in obesity and insulin resistance". Journal of Internal Medicine. 245 (6): 621–25. doi:10.1046/j.1365-2796.1999.00490.x. PMID 10395191. S2CID 58332116.
- ↑ Whitney, Ellie and Ralfes, R. Sharon. 2011. Understanding Nutrition. Wadsworth Cengage Learning: Belmont, CA
- 1 2 Gatta-Cherifi, Blandine; Cota, Daniela (2015). "Endocannabinoids and Metabolic Disorders". Endocannabinoids. Handbook of Experimental Pharmacology. Vol. 231. pp. 367–91. doi:10.1007/978-3-319-20825-1_13. ISBN 978-3-319-20824-4. PMID 26408168.
The endocannabinoid system (ECS) is known to exert regulatory control on essentially every aspect related to the search for, and the intake, metabolism and storage of calories, and consequently it represents a potential pharmacotherapeutic target for obesity, diabetes and eating disorders. ... recent research in animals and humans has provided new knowledge on the mechanisms of actions of the ECS in the regulation of eating behavior, energy balance, and metabolism. In this review, we discuss these recent advances and how they may allow targeting the ECS in a more specific and selective manner for the future development of therapies against obesity, metabolic syndrome, and eating disorders.
- 1 2 3 Vemuri VK, Janero DR, Makriyannis A (March 2008). "Pharmacotherapeutic targeting of the endocannabinoid signaling system: drugs for obesity and the metabolic syndrome". Physiology & Behavior. 93 (4–5): 671–86. doi:10.1016/j.physbeh.2007.11.012. PMC 3681125. PMID 18155257.
The etiology of many appetitive disorders is characterized by a pathogenic component of reward-supported craving, be it for substances of abuse (including alcohol and nicotine) or food. Such maladies affect large numbers of people as prevalent socioeconomic and healthcare burdens. Yet in most instances drugs for their safe and effective pharmacotherapeutic management are lacking despite the attendant medical needs, collateral adverse physical and psychological effects, and enormous global market potential. The endocannabinoid signaling system plays a critical role in motivational homeostasis as a conduit for reward stimuli and a positive modulator of brain reward circuits. Endocannabinoid-system hyperactivity through CB1 receptor transmission is considered contributory to a range of appetitive disorders and, hence, is a major focus of contemporary pharmaceutical research.
- 1 2 3 4 Turcotte C, Chouinard F, Lefebvre JS, Flamand N (June 2015). "Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites". Journal of Leukocyte Biology. 97 (6): 1049–70. doi:10.1189/jlb.3RU0115-021R. PMID 25877930. S2CID 206999921.
- ↑ Fukuchi S, Hamaguchi K, Seike M, Himeno K, Sakata T, Yoshimatsu H (June 2004). "Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats". Experimental Biology and Medicine. 229 (6): 486–93. doi:10.1177/153537020422900606. PMID 15169967. S2CID 20966659.
- ↑ Di Marzo V, Fontana A, Cadas H, et al. (Dec 1994). "Formation and inactivation of endogenous cannabinoid anandamide in central neurons". Nature (Submitted manuscript). 372 (6507): 686–91. Bibcode:1994Natur.372..686D. doi:10.1038/372686a0. PMID 7990962. S2CID 4341716. Archived from the original on 2021-07-09. Retrieved 2022-05-17.
- ↑ Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC (October 2009). "Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity" (PDF). Circulation. 120 (16): 1640–45. doi:10.1161/CIRCULATIONAHA.109.192644. PMID 19805654. Archived (PDF) from the original on 2018-04-21. Retrieved 2022-05-17.
- 1 2 3 Alberti, George; Zimmet, Paul; Shaw, Johnathan (2006). Grundy, Scott M. (ed.). IDF Consensus Worldwide Definition of the Metabolic Syndrome (PDF) (Report). Brussels, Belgium: International Diabetes Federation. Archived from the original on 2012-09-16.
- ↑ Alberti, KGMM; Zimmet (1999). "Definition, Diagnosis, and Classification of Diabetes Mellitus and its Complications" (PDF). World Health Organization. pp. 32–33. Archived from the original (PDF) on 21 August 2014. Retrieved 25 March 2013.
- ↑ Expert Panel On Detection, Evaluation (May 2001). "Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)". JAMA. 285 (19): 2486–97. doi:10.1001/jama.285.19.2486. PMID 11368702.
- ↑ Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C (January 2004). "Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition". Circulation. 109 (3): 433–38. doi:10.1161/01.CIR.0000111245.75752.C6. PMC 1880831. PMID 14744958.
- ↑ Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F (October 2005). "Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement". Circulation. 112 (17): 2735–52. doi:10.1161/CIRCULATIONAHA.105.169404. PMID 16157765. S2CID 7571079.
- ↑ Kogiso T, Moriyoshi Y, Shimizu S, Nagahara H, Shiratori K (2009). "High-sensitivity C-reactive protein as a serum predictor of nonalcoholic fatty liver disease based on the Akaike Information Criterion scoring system in the general Japanese population". Journal of Gastroenterology. 44 (4): 313–21. doi:10.1007/s00535-009-0002-5. PMID 19271113. S2CID 1193178.
- ↑ Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT (February 2011). "Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies". International Journal of Epidemiology. 40 (1): 189–207. doi:10.1093/ije/dyq158. PMID 20870782.
- ↑ Lakka TA, Laaksonen DE (February 2007). "Physical activity in prevention and treatment of the metabolic syndrome". Applied Physiology, Nutrition, and Metabolism. 32 (1): 76–88. doi:10.1139/h06-113. PMID 17332786.
- ↑ Feldeisen SE, Tucker KL (February 2007). "Nutritional strategies in the prevention and treatment of metabolic syndrome". Applied Physiology, Nutrition, and Metabolism. 32 (1): 46–60. doi:10.1139/h06-101. PMID 17332784.
- ↑ James PT, Rigby N, Leach R (February 2004). "The obesity epidemic, metabolic syndrome and future prevention strategies". European Journal of Cardiovascular Prevention and Rehabilitation. 11 (1): 3–8. doi:10.1097/01.hjr.0000114707.27531.48. PMID 15167200. S2CID 36797932.
- ↑ Elwood PC, Pickering JE, Fehily AM (August 2007). "Milk and dairy consumption, diabetes and the metabolic syndrome: the Caerphilly prospective study". Journal of Epidemiology and Community Health. 61 (8): 695–98. doi:10.1136/jech.2006.053157. PMC 2652996. PMID 17630368.
- ↑ Snijder MB, van der Heijden AA, van Dam RM, Stehouwer CD, Hiddink GJ, Nijpels G, Heine RJ, Bouter LM, Dekker JM (April 2007). "Is higher dairy consumption associated with lower body weight and fewer metabolic disturbances? The Hoorn Study". The American Journal of Clinical Nutrition. 85 (4): 989–95. doi:10.1093/ajcn/85.4.989. PMID 17413097.
- ↑ Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H (October 2015). "Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis". The American Journal of Clinical Nutrition. 102 (4): 922–32. doi:10.3945/ajcn.115.113613. PMC 4588744. PMID 26269362.
- ↑ Srikanthan, K; Feyh, A; Visweshwar, H; Shapiro, J. I; Sodhi, K (2016). "Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population". International Journal of Medical Sciences. 13 (1): 25–38. doi:10.7150/ijms.13800. PMC 4716817. PMID 26816492.
- ↑ Feinman, R. D; Pogozelski, W. K; Astrup, A; Bernstein, R. K; Fine, E. J; Westman, E. C; Accurso, A; Frassetto, L; Gower, B. A; McFarlane, S. I; Nielsen, J. V; Krarup, T; Saslow, L; Roth, K. S; Vernon, M. C; Volek, J. S; Wilshire, G. B; Dahlqvist, A; Sundberg, R; Childers, A; Morrison, K; Manninen, A. H; Dashti, H. M; Wood, R. J; Wortman, J; Worm, N (2015). "Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base". Nutrition. 31 (1): 1–13. doi:10.1016/j.nut.2014.06.011. PMID 25287761. Archived from the original on 2018-07-17. Retrieved 2022-05-17.
- ↑ Ford ES, Li C, Zhao G (September 2010). "Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US". Journal of Diabetes. 2 (3): 180–93. doi:10.1111/j.1753-0407.2010.00078.x. PMID 20923483. S2CID 5145131. Archived from the original on 2022-05-18. Retrieved 2022-05-17.
- 1 2 Ford ES, Giles WH, Mokdad AH (October 2004). "Increasing prevalence of the metabolic syndrome among u.s. Adults". Diabetes Care. 27 (10): 2444–49. doi:10.2337/diacare.27.10.2444. PMID 15451914.
- ↑ Mozumdar A, Liguori G (January 2011). "Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006". Diabetes Care. 34 (1): 216–19. doi:10.2337/dc10-0879. PMC 3005489. PMID 20889854.
- ↑ Kamel, M.; Smith, B. T.; Wahi, G.; Carsley, S.; Birken, C. S.; Anderson, L. N. (September 17, 2018). "Continuous cardiometabolic risk score definitions in early childhood: a scoping review: Cardiometabolic risk definitions". Obesity Reviews. 19 (12): 1688–1699. doi:10.1111/obr.12748. PMID 30223304. S2CID 52291692. Retrieved 17 November 2020.
- ↑ Chiarelli, Francesco; Mohn, Angelika (October 2017). "Early diagnosis of metabolic syndrome in children". The Lancet Child & Adolescent Health. 1 (2): 86–88. doi:10.1016/S2352-4642(17)30043-3. PMID 30169210. Archived from the original on 3 June 2022. Retrieved 17 November 2020.
- ↑ Joslin, Elliot P. (1921). "The Prevention of Diabetes Mellitus". JAMA. 76 (2): 79–84. doi:10.1001/jama.1921.02630020001001.
- ↑ Kylin E. [Studies of the hypertension-hyperglycemia-hyperuricemia syndrome] (German). Zentralbl Inn Med 1923; 44: 105–27.
- ↑ Vague J. La diffférenciacion sexuelle, facteur déterminant des formes de l'obésité. Presse Med 1947;30:339-40.
- ↑ Avogaro, Piero; Crepaldi, Gaetano; Enzi, Giuliano; Tiengo, Antonio (1967). "Associazione di iperlipemia, diabete mellito e obesita' di medio grado" [Association of hyperlipemia, diabetes mellitus and middle-degree obesity]. Acta Diabetologica Latina (in italiano). 4 (4): 572–90. doi:10.1007/BF01544100. S2CID 25839940.
- ↑ Haller H (April 1977). "[Epidermiology and associated risk factors of hyperlipoproteinemia]". Zeitschrift für Sie Gesamte Innere Medizin und Ihre Grenzgebiete. 32 (8): 124–28. PMID 883354.
- ↑ Singer P (May 1977). "[Diagnosis of primary hyperlipoproteinemias]". Zeitschrift für die Gesamte Innere Medizin und Ihre Grenzgebiete. 32 (9): 129–33. PMID 906591.
- ↑ Phillips GB (July 1978). "Sex hormones, risk factors and cardiovascular disease". The American Journal of Medicine. 65 (1): 7–11. doi:10.1016/0002-9343(78)90685-X. PMID 356599.
- ↑ Phillips GB (April 1977). "Relationship between serum sex hormones and glucose, insulin and lipid abnormalities in men with myocardial infarction". Proceedings of the National Academy of Sciences of the United States of America. 74 (4): 1729–33. Bibcode:1977PNAS...74.1729P. doi:10.1073/pnas.74.4.1729. PMC 430867. PMID 193114.
- ↑ Reaven GM (December 1988). "Banting lecture 1988. Role of insulin resistance in human disease". Diabetes. 37 (12): 1595–607. doi:10.2337/diabetes.37.12.1595. PMID 3056758.
External links
| Classification | |
|---|---|
| External resources |