Caspofungin
 | |
| Names | |
|---|---|
| Pronunciation | /ˌkæspoʊˈfʌndʒɪn/ KAS-poh-FUN-jin |
| Trade names | Cancidas |
IUPAC name
| |
| Clinical data | |
| Main uses | Fungal infections (candidiasis, aspergillosis)[1] |
| Side effects | Diarrhea, fever, liver problems, low potassium[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 100% (intravenous use only) |
| Protein binding | ~97% |
| Metabolism | Liver |
| Elimination half-life | 9–11 hours |
| Excretion | Urine (41%), feces (35%) |
| Chemical and physical data | |
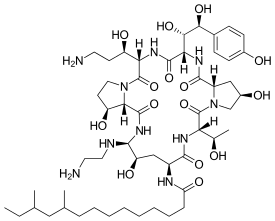
| Formula | C52H88N10O15 |
| Molar mass | 1093.331 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Caspofungin, sold under the brand name Cancidas, is a medication used to treat fungal infections such as candidiasis and aspergillosis.[1] It may be used in people with low neutrophils.[1] It is given by injection into a vein.[1]
Common side effects include diarrhea, fever, liver problems, and low potassium.[2] Other side effects may include anaphylaxis and Stevens-Johnson syndrome.[2] Use in pregnancy may harm the baby.[2] It is a antifungal of the lipopeptide and echinocandin class.[3] It works by interfering with the cell wall of the fungus.[3]
Caspofungin was approved for medical use in the United States and Europe in 2001.[3][4] It is on the World Health Organization's List of Essential Medicines as an alternative to micafungin.[5] It is available as a generic medication.[1] In the United Kingdom it costs about £50 as of 2021.[1] This amount in the United States is about 60 USD.[6]
Medical uses
Caspofungin acetate for injection was originally approved by both the Food and Drug Administration (FDA), in the U.S., and the EMEA, in Europe, in 2001.
Its currently approved therapeutic indications by both organisations include the empirical therapy of presumed fungal infections in febrile, neutropenic adult patients and for salvage therapy in patients treatment of invasive aspergillosis in adult patients whose disease is refractory to, or who are intolerant of, other antifungal agents (i.e., conventional or lipid formulations of amphotericin B and/or itraconazole). Additionally, the FDA approval includes indication for the treatment of candidemia and some specific Candida infections (intra-abdominal abscesses, peritonitis, pleural cavity infections, and esophagitis) and the EMEA approval includes indication for the treatment of general invasive candidiasis in adult patients.
About 36% of patients refractory to other therapies responded well to caspofungin therapy, while even 70% of patients intolerant to other therapies were classified as responders. Direct comparative studies to other drugs in the treatment of invasive aspergillosis have so far not been undertaken.
Elderly
Ordinarily, no dose adjustments are necessary, however, greater sensitivity of some older individuals cannot be ruled out.[7]
Children
Caspofungin is FDA approved for pediatric patients 3 months and older.[7] Dosing is based on body surface area (BSA) as calculated by the Mosteller formula.[8]
Spectrum of activity
Caspofungin has been effective in treating fungal infections caused by Aspergillus and Candida species. It is a member of the echinocandin family, a new class of antifungal agents with broad spectrum of activity against all Candida species. In comparison to treatment with either fluconazole or Amphotericin B, all three drugs in this class have been demonstrated to be highly effective or superior in well-defined clinical settings including invasive Candida infections, Candida oesophagitis and candidaemia. Higher minimum inhibitory concentration (MIC) of these agents has been observed against C. parapsilosis and C. guilliermondii.[9]
The following summarizes MIC susceptibility for a few medically significant organisms.[10]
- Candida albicans 0.015 — 16 μg/mL
- Candida krusei 0.03 — 8 μg/mL
- Cryptococcus neoformans — 16 μg/mL
Dosage
An initial dose of 70 mg by intravenous infusion is given followed by 50 mg intravenous daily.[1] If no response is seen or if inducers of caspofungin clearance (see above) are coadministered the daily dose may be increased to 70 mg. An infusion should take approximately 1 hour.
The mean duration of therapy in previous studies was 34 days. Some patients were even healed by a one-day treatment. However, a few patients were treated for as long as 162 days and tolerated the drug well, indicating that longtime use may be indicated and tolerated favourably in complicated cases of aspergillosis. Generally, the duration of treatment is dictated by the severity of the disease, the clinical response, and the improvement of immunocompetence in immunocompromised patients.
- Cancidas 50 mg for intravenous infusion (manufacturer Merck)
- Cancidas 70 mg for intravenous infusion (manufacturer Merck)
- Brand names in countries other than the U.S. may vary.
Contraindications
Known hypersensitivity to caspofungin acetate or any other ingredient contained in the formulation contraindicate its use.
Side effects
Compared to amphotericin B, caspofungin seems to have a relatively low incidence of side effects. In clinical studies and postmarketing reports, the side effects seen in 1% or more of the patients were as follows:
- Gastrointestinal system: nausea, vomiting, abdominal pain, and diarrhea
- Central nervous system: headache
- Whole body: fever, phlebitis or thrombophlebitis, complications at the intravenous cannulation site (e.g. induration), unspecified pain, flu-like syndrome, myalgia, chills, and paresthesia
- Respiratory: dyspnea
- Kidney: increased plasma creatinine
- Hematological: anemia
- Electrolytes: hypokalemia
- Liver: increased liver enzymes (asymptomatic)
- Hypersensitivity: rash, facial edema, pruritus
- Other: tachycardia
Additionally, infrequent cases of symptomatic liver damage, peripheral edema and swelling, and hypercalcemia have been seen. One case of anaphylaxis (severe allergic reaction) has also been noted.
- Liver
The concomitant use of caspofungin and cyclosporine in healthy volunteers led to a more frequent increase of liver enzymes (ALT=SGPT and AST=SGOT) than noted with cyclosporine alone. Combination treatment is only indicated if the potential benefit for the patient outweighs the potential risk.
Dosage reduction in patients with moderately impaired liver function is recommended. No clinical data exist regarding the use of caspofungin in patients with severely impaired liver function.
- Sensitivity reactions
Reactions due to histamine release (rash, facial swelling, pruritus, sensation of warmth and one case of anaphylaxis) have been seen. Health-care providers should carefully watch for these reactions.
- Drug resistance
In a few patients with infections caused by Candida albicans, mutants with reduced sensitivity to caspofungin have been noticed. Currently there are no data regarding development of resistance in other fungi than C. albicans.
Pregnancy and breastfeeding
Caspofungin has been shown in animal studies to have embroyotoxic properties, and therefore has been assigned to class C. It should only be given to pregnant women if the benefit to the mother clearly outweighs the potential risk to her fetus.
The drug is found in the milk of lactating rats, but it is not known whether this is seen in humans. Thus, lactating women should be treated cautiously.
Resistance
Resistance in C. albicans has been described, but is currently still rare. The mechanism is probably a point mutation in the (1→3)-β-D-glucan synthase gene.[11]
Interactions
- Cyclosporin: see under hepatic effects
- Tacrolimus: potential pharmacokinetic interactions
- Other systemic antimycotic agents: with amphotericin B, itraconazole and mycophenolate, no interactions have been seen
- Inducers of drug clearance (e.g. carbamazepine, phenytoin, rifampin, dexamethasone): consider 70 mg intravenous as maintenance dose instead of 50 mg
Metabolism
Slowly metabolized by peptide hydrolysis and N-acetylation in liver. Therefore, in case of liver impairment the dose needs to be reduced. Caspofungin also undergoes spontaneous chemical degradation to an open-ring peptide compound, L-747969. Additional metabolism involves hydrolysis into constitutive amino acids and their derivatives, including dihydroxyhomotyrosine and N-acetyl-dihydroxyhomotyrosine.[7]
Chemistry
It is a lipopeptide antifungal. It is a member of a new class of antifungals termed the echinocandins. It works by inhibiting the enzyme (1→3)-β-D-glucan synthase and thereby disturbing the integrity of the fungal cell wall.
Chemically it is known as (4R,5S)-5-[(2-Aminoethyl)amino]-N2-(10,12-dimethyltetradecanoyl)-4-hydroxy-L-ornithyl-L-threonyl-trans-4-hydroxy-L-prolyl-(S)-4-hydroxy-4-(p-hydroxyphenyl)-L-threonyl-threo-3-hydroxy-L-ornithyl-trans-3-hydroxy-L-proline cyclic (6→1)-peptide
[12]: 185 and 1-[(4R,5S)-5-[(2-Aminoethyl)amino]-N2-(10,12-dimethyl-1-oxotetradecyl)-4-hydroxy-L-ornithine]-5-[(3R)-3-hydroxy-L-ornithine] pneumocandin B0[7]: 13
Semisynthesis
Caspofungin is semisynthesized from pneumocandin B0, a fermentation product of Glarea lozoyensis.[13]
History
Caspofungin was the first inhibitor of fungal (1→3)-β-D-glucan synthesis to be approved by the United States Food and Drug Administration.[13] It was discovered by James Balkovec, Regina Black and Frances A. Bouffard.[14]
References
- 1 2 3 4 5 6 7 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 632. ISBN 978-0857114105.
- 1 2 3 4 "DailyMed - CASPOFUNGIN ACETATE injection". dailymed.nlm.nih.gov. Archived from the original on 1 April 2021. Retrieved 30 December 2021.
- 1 2 3 "Caspofungin Monograph for Professionals". Drugs.com. Archived from the original on 23 April 2021. Retrieved 30 December 2021.
- ↑ "Cancidas (previously Caspofungin MSD)". Archived from the original on 10 November 2021. Retrieved 30 December 2021.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ "Caspofungin Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 30 December 2021.
- 1 2 3 4 "Cancidas (caspofungin acetate) for Injection, for Intravenous Use. Full Prescribing Information" (PDF). Merck & Co., Inc., Whitehouse Station, NJ 08889, USA. Archived (PDF) from the original on 2 October 2016. Retrieved 11 November 2016.
- ↑ Mosteller RD (October 1987). "Simplified calculation of body-surface area". The New England Journal of Medicine. 317 (17): 1098. doi:10.1056/NEJM198710223171717. PMID 3657876.
- ↑ Kofla G, Ruhnke M (April 2011). "Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature". European Journal of Medical Research. 16 (4): 159–66. doi:10.1186/2047-783X-16-4-159. PMC 3352072. PMID 21486730.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2013-08-13.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Baixench MT, Aoun N, Desnos-Ollivier M, Garcia-Hermoso D, Bretagne S, Ramires S, et al. (June 2007). "Acquired resistance to echinocandins in Candida albicans: case report and review". The Journal of Antimicrobial Chemotherapy. 59 (6): 1076–83. doi:10.1093/jac/dkm095. PMID 17468115.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). RECOMMENDED International Nonproprietary names (Rec.INN): List 42" (PDF). World Health Organization. 1999. Archived (PDF) from the original on 18 May 2016. Retrieved 11 November 2016.
- 1 2 Deresinski SC, Stevens DA (June 2003). "Caspofungin". Clinical Infectious Diseases. 36 (11): 1445–57. doi:10.1086/375080. PMID 12766841.
- ↑ "Patent Covering Caspofungin". Archived from the original on 7 April 2015. Retrieved 18 March 2015.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- U.S. Full Prescribing Information Archived 2021-04-01 at the Wayback Machine
- EMA on Cancidas (Summary of Product Characteristics) Archived 2021-04-14 at the Wayback Machine