Aspergillosis
| Aspergillosis | |
|---|---|
.JPG.webp) | |
| Chest X-ray: widespread rounded lung nodules throughout both lungs | |
| Pronunciation |
|
| Specialty | Infectious disease |
| Symptoms | Depend on the type of disease[1] |
| Types | Allergic bronchopulmonary aspergillosis (ABPA); aspergilloma; chronic pulmonary aspergillosis (CPA); allergic sinusitis; infection of the skin[2] |
| Causes | Aspergillus fumigatus, Aspergillus flavus, others[3] |
| Risk factors | Lung disease, poor immune function[4] |
| Differential diagnosis | Mucormycosis, nocardiosis, pulmonary eosinophilia, other types of pneumonia[4] |
| Treatment | Antifungal medication, surgery[5] |
| Frequency | Uncommon[4] |
Aspergillosis is a fungal disease caused by Aspergillus, a common mold.[6] There are several types including: allergic bronchopulmonary aspergillosis (ABPA), inflammation of the lungs and allergic symptoms without infection; aspergilloma, an area of fungus in the lungs or sinuses; chronic pulmonary aspergillosis (CPA), formation of a cavity within the lungs from infection; allergic sinusitis; and infection of the skin.[2] Symptoms depend on the type of disease.[1]
The most frequently involved species are Aspergillus fumigatus and Aspergillus flavus.[3] Most people breath in Aspergillus spores daily without getting sick.[2] Illness generally only occurs in people with lung diseases such as asthma, cystic fibrosis, COPD, sarcoidosis, or tuberculosis; or those with poor immune function such as during chemotherapy, stem cell transplantation, HIV/AIDS, or long term use of steroids.[4] It does not spread between people.[7] Diagnosis may involve medical imaging, tissue biopsy, or blood tests.[8]
Treatment of allergic disease of the lungs or sinuses may involve the use of itraconazole and steroids.[5] An aspergilloma may be managed with surgery or antifungal medications.[5] Invasive disease may be treated with voriconazole, amphotericin B, or posaconazole.[5] Among those with invasive disease, death may occur as a result in 25 to 60%.[9]
Aspergillosis is uncommon.[4] Allergic bronchopulmonary aspergillosis is estimated to affect about 2.5 million people while chronic pulmonary aspergillosis affects about 3 million globally.[4] Outbreaks of disease have been documented in hospitals.[10] Invasive forms of disease are estimated to result in around 600,000 deaths a year.[9] It can affect birds and other animals.[11] The mold was first described in 1729 by Pier Antonio Micheli, an Italian priest.[12][13]
Classification
Aspergillosis can be classified generally as allergic, chronic and invasive, or more specifically as allergic bronchopulmonary aspergillosis, allergic aspergillus sinusitis, invasive aspergillosis, cutaneous (skin) aspergillosis, and chronic pulmonary aspergillosis.[4]
Signs and symptoms
Signs and symptoms vary according to the type of aspergillosis.[14] Generally, common symptoms include wheezing and coughing of blood.[15]
Allergic bronchopulmonary aspergillosis can appear similar to asthma and present with wheeze, cough, difficulty breathing, and less commonly fever.[14] Allergic aspergillosis affecting the sinuses presents with blocked or runny nose, headache and loss of smell.[14] Azole-Resistant Aspergillus fumigatus and Aspergilloma (fungal ball) may present with cough with or without blood and shortness of breath.[14] Chronic pulmonary aspergillosis, with longterm involvement of the lungs may have additional weight loss and tiredness.[14]
Invasive aspergillosis typically occurs in people who have other medical conditions and distinguishing which symptoms are from which condition may be difficult.[14] It may present with chest pain, fever, cough with or without blood, and difficulty breathing.[14] Poorly controlled aspergillosis can disseminate through the blood stream to cause widespread organ damage. Symptoms include fever, chills, shock, delirium, seizures, and blood clots. The person may develop kidney failure, liver failure (causing jaundice), and breathing difficulties. Death can occur quickly.
Aspergillosis of the ear canal causes itching and occasionally pain. Fluid draining overnight from the ear may leave a stain on the pillow. Aspergillosis of the sinuses causes a feeling of congestion and sometimes pain or discharge. It can extend beyond the sinuses.[16]
Skin
Aspergillosis of skin can occur when Aspergillus enters the body through a break in the skin.[14] Nodules and plaques may appear purplish, before developing an ulcer with tissue death and black eschar.[17]
Causes

Allergic bronchopulmonary aspergillosis is caused by a hypersensitivity reaction to the Aspergillus species.[3] The most common causative species is Aspergillus fumigatus.[18]
Risk factors
Most people breath in Aspergillus spores daily without getting sick.[2] Illness generally only occurs in people with lung diseases such as asthma, cystic fibrosis, COPD, sarcoidosis, or tuberculosis.[4] Invasive aspergillosis is most common in people who have weak immune systems, such as people who have had a transplant, particularly a hematopoietic stem cell transplantation, or are on chemotherapy, and those who have conditions such as HIV/AIDS,leukaemia. These conditions tend to result in low white cells, which prevents the body in mounting immune responses against the fungi and thereby allowing uncontrolled growth of the fungal filaments. The immune system may be weakened by taking medications such as corticosteroids.[19]
COVID-19-associated pulmonary aspergillosis has been reported in people who have severe COVID-19 and have been on ventilators.[20]
Diagnosis
Imaging
The extent of infection may be seen on X-ray or CT scan. On chest X-ray and CT, pulmonary aspergillosis classically manifests as a halo sign, and later, an air crescent sign.[21]
_(Radiopaedia_5745-7413_Frontal_1).jpg.webp) Chest X-ray: mass overlying the left hilum
Chest X-ray: mass overlying the left hilum.jpeg.webp) Large cavity mass lesion seen right upper lung
Large cavity mass lesion seen right upper lung.jpg.webp) CT scan lungs: multiple lung lesions with ground-glass opacity suggesting haemorrhage
CT scan lungs: multiple lung lesions with ground-glass opacity suggesting haemorrhage
Culture and biopsy
Whenever possible, a doctor sends a sample of infected material to a laboratory to confirm identification of the fungus.
On microscopy, Aspergillus species are reliably demonstrated by silver stains, e.g., Gridley stain or Gomori methenamine-silver.[22] These give the fungal walls a gray-black colour. The hyphae of Aspergillus species range in diameter from 2.5 to 4.5 µm. They have septate hyphae,[23] but these are not always apparent, and in such cases they may be mistaken for Zygomycota.[22] Aspergillus hyphae tend to have dichotomous branching that is progressive and primarily at acute angles of around 45°.[22]
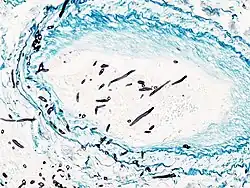 Angioinvasive pulmonary aspergillosis
Angioinvasive pulmonary aspergillosis_invasive_type.jpg.webp) Angioinvasive pulmonary aspergillosis (closeup)
Angioinvasive pulmonary aspergillosis (closeup)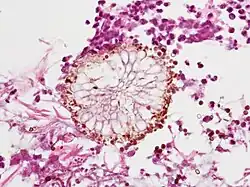 Aspergillus vesicle (HE stain)
Aspergillus vesicle (HE stain)_(Radiopaedia_8675-9486_B_1).jpg.webp) Angioinvasive aspergillosis demonstrating the gross pathology equivalent of the CT ground glass halo
Angioinvasive aspergillosis demonstrating the gross pathology equivalent of the CT ground glass halo
Non-culture-based
Microbiological cultures are not very sensitive.[24] Non-culture-based Aspergillus antigen detection tests can make the diagnosis in a noninvasive way.[24]
False-positive Aspergillus galactomannan tests have been found in patients on intravenous treatment with some antibiotics or fluids containing gluconate or citric acid such as some transfusion platelets, parenteral nutrition, or PlasmaLyte.
Screening
Evidence suggests polymerase chain reaction (PCR) tests have moderate diagnostic accuracy when used for screening for invasive aspergillosis in high risk groups.[25]
Prevention
Prevention of aspergillosis involves a reduction of mold exposure via environmental infection-control. Antifungal prophylaxis can be given to high-risk patients. Posaconazole is often given as prophylaxis in severely immunocompromised patients.[26]
Treatment
The current medical treatments for aggressive invasive aspergillosis include voriconazole and liposomal amphotericin B in combination with surgical debridement.[27] For the less aggressive allergic bronchopulmonary aspergillosis, findings suggest the use of oral steroids for a prolonged period of time, preferably for 6–9 months in allergic aspergillosis of the lungs. Itraconazole is given with the steroids, as it is considered to have a "steroid-sparing" effect, causing the steroids to be more effective, allowing a lower dose.[28] Other drugs used, such as amphotericin B, caspofungin (in combination therapy only), flucytosine (in combination therapy only), or itraconazole,[29][30] are used to treat this fungal infection. However, a growing proportion of infections are resistant to the triazoles.[31] A. fumigatus, the most commonly infecting species, is intrinsically resistant to fluconazole.[32]
Epidemiology
Aspergillosis is thought to affect more than 14 million people worldwide,[33] with allergic bronchopulmonary aspergillosis (ABPA, >4 million), severe asthma with fungal sensitization (>6.5 million), and chronic pulmonary aspergillosis (CPA, ∼3 million) being considerably more prevalent than invasive aspergillosis (IA, >300,000). Other common conditions include Aspergillus bronchitis, Aspergillus rhinosinusitis (many millions), otitis externa, and Aspergillus onychomycosis (10 million).[34][35] Alterations in the composition and function of the lung microbiome and mycobiome have been associated with an increasing number of chronic pulmonary diseases such as COPD, cystic fibrosis, chronic rhinosinusitis and asthma.[36]
History
The mold was first described in 1729 by Pier Antonio Micheli, an Italian priest.[12]
Society and culture
During the COVID-19 pandemic, COVID-19-associated pulmonary aspergillosis was reported in some people who had been admitted to hospital and received longterm steroid treatment.[37]
Other animals
While relatively rare in humans, aspergillosis is a common and dangerous infection in birds, particularly in pet parrots. Mallards and other ducks are particularly susceptible, as they often resort to poor food sources during bad weather. Captive raptors, such as falcons and hawks, are susceptible to this disease if they are kept in poor conditions and especially if they are fed pigeons, which are often carriers of "asper". It can be acute in chicks, but chronic in mature birds.
In the United States, aspergillosis has been the culprit in several rapid die-offs among waterfowl. From 8 December until 14 December 2006, over 2,000 mallards died near Burley, Idaho, an agricultural community about 150 miles southeast of Boise. Mouldy waste grain from the farmland and feedlots in the area is the suspected source. A similar aspergillosis outbreak caused by mouldy grain killed 500 mallards in Iowa in 2005.
While no connection has been found between aspergillosis and the H5N1 strain of avian influenza (commonly called "bird flu"), rapid die-offs caused by aspergillosis can spark fears of bird flu outbreaks. Laboratory analysis is the only way to distinguish bird flu from aspergillosis.
In dogs, aspergillosis is an uncommon disease typically affecting only the nasal passages (nasal aspergillosis). This is much more common in dolicocephalic breeds. It can also spread to the rest of the body; this is termed disseminated aspergillosis and is rare, usually affecting individuals with underlying immune disorders.
In 2019, an outbreak of aspergillosis struck the rare kakapo, a large flightless parrot endemic to New Zealand. By June the disease had killed seven of the birds, whose total population at the time was only 142 adults and 72 chicks. One fifth of the population was infected with the disease and the entire species was considered at risk of extinction.[38]
See also
References
- 1 2 "Symptoms of Aspergillosis". www.cdc.gov. CDC. 2 February 2021. Archived from the original on 18 June 2021. Retrieved 8 July 2021.
- 1 2 3 4 "About Aspergillosis". www.cdc.gov. CDC. 2 February 2021. Archived from the original on 27 June 2021. Retrieved 8 July 2021.
- 1 2 3 Thornton, Christopher R. (2020). "1. Detection of the 'Big Five' mold killers of humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes". In Gadd, Geoffrey M.; Sariaslani, Sima (eds.). Advances in Applied Microbiology. Academic Press. pp. 1–7. ISBN 978-0-12-820703-1. Archived from the original on 2021-05-25. Retrieved 2021-05-25.
- 1 2 3 4 5 6 7 8 "Aspergillosis". NORD (National Organization for Rare Disorders). Archived from the original on 26 May 2021. Retrieved 26 May 2021.
- 1 2 3 4 "Treatment for Aspergillosis". www.cdc.gov. CDC. 27 January 2021. Archived from the original on 30 June 2021. Retrieved 8 July 2021.
- ↑ "Aspergillosis | Types of Fungal Diseases | Fungal Diseases". www.cdc.gov. CDC. 10 May 2021. Archived from the original on 16 July 2021. Retrieved 7 July 2021.
- ↑ "Aspergillosis Risk & Prevention". www.cdc.gov. 27 January 2021. Archived from the original on 31 May 2021. Retrieved 8 July 2021.
- ↑ "Diagnosis and Testing for Aspergillosis | Aspergillosis". www.cdc.gov. 29 April 2021. Archived from the original on 28 June 2021. Retrieved 8 July 2021.
- 1 2 Zhou, Xuedong; Li, Yuqing (6 January 2021). Atlas of Oral Microbiology: From Healthy Microflora to Disease. Springer Nature. p. 282. ISBN 978-981-15-7899-1. Archived from the original on 27 August 2021. Retrieved 8 July 2021.
- ↑ "Aspergillosis Statistics". www.cdc.gov. 5 December 2019. Archived from the original on 13 May 2021. Retrieved 8 July 2021.
- ↑ Thomas, Nancy J.; Hunter, D. Bruce; Atkinson, Carter T. (9 January 2008). Infectious Diseases of Wild Birds. John Wiley & Sons. p. 364. ISBN 978-0-470-34436-1. Archived from the original on 27 August 2021. Retrieved 7 July 2021.
- 1 2 "Etymologia: Aspergillus". Emerging Infectious Diseases. 12 (3): 415. March 2006. doi:10.3201/eid1203.ET1203. ISSN 1080-6040. PMC 3291468. Archived from the original on 2021-01-17. Retrieved 2021-07-08.
- ↑ Hospenthal, Duane R.; Rinaldi, Michael G. (12 May 2015). Diagnosis and Treatment of Fungal Infections. Springer. p. 129. ISBN 978-3-319-13090-3. Archived from the original on 27 August 2021. Retrieved 8 July 2021.
- 1 2 3 4 5 6 7 8 "Symptoms of Aspergillosis | Aspergillosis | Types of Fungal Diseases | Fungal Diseases | CDC". www.cdc.gov. 2 February 2021. Archived from the original on 18 June 2021. Retrieved 23 May 2021.
- ↑ "ICD-11 - ICD-11 for Mortality and Morbidity Statistics". icd.who.int. Archived from the original on 1 August 2018. Retrieved 22 May 2021.
- ↑ Ederies A, Chen J, Aviv RI, Pirouzmand F, Bilbao JM, Thompson AL, Symons SP (May 2010). "Aspergillosis of the Petrous Apex and Meckel's Cave". Skull Base. 20 (3): 189–92. doi:10.1055/s-0029-1246229. PMC 3037105. PMID 21318037.
- ↑ Johnstone, Ronald B. (2017). "25. Mycoses and Algal infections". Weedon's Skin Pathology Essentials (2nd ed.). Elsevier. p. 463. ISBN 978-0-7020-6830-0. Archived from the original on 2023-06-30. Retrieved 2023-06-20.
- ↑ Willinger, Birgit (2019). "1. What is the target? Clinical mycology and diagnostics". In Elisabeth Presterl (ed.). Clinically Relevant Mycoses: A Practical Approach. Springer. pp. 3–19. ISBN 978-3-319-92299-7. Archived from the original on 2021-06-02. Retrieved 2021-06-01.
- ↑ Dagenais TR, Keller NP (July 2009). "Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis". Clinical Microbiology Reviews. 22 (3): 447–65. doi:10.1128/CMR.00055-08. PMC 2708386. PMID 19597008.
- ↑ "Fungal Diseases and COVID-19 | CDC". www.cdc.gov. 7 June 2021. Archived from the original on 21 June 2021. Retrieved 9 August 2021.
- ↑ Curtis AM, Smith GJ, Ravin CE (October 1979). "Air crescent sign of invasive aspergillosis". Radiology. 133 (1): 17–21. doi:10.1148/133.1.17. PMID 472287.
- 1 2 3 Kradin RL, Mark EJ (April 2008). "The pathology of pulmonary disorders due to Aspergillus spp". Archives of Pathology & Laboratory Medicine. 132 (4): 606–14. doi:10.1043/1543-2165(2008)132[606:TPOPDD]2.0.CO;2 (inactive 2021-01-16). PMID 18384212. Archived from the original on 2022-02-08. Retrieved 2021-03-01.
{{cite journal}}: CS1 maint: DOI inactive as of January 2021 (link) - ↑ "Mycoses: Aspergillosis". Mycology Online. Archived from the original on 7 December 2008. Retrieved 2008-12-10.
- 1 2 "The selection and use of essential in vitro diagnostics - 2020". www.who.int. World Health Organization. pp. 55–66. Archived from the original on 20 August 2021. Retrieved 20 August 2021.
- ↑ Cruciani M, Mengoli C, Barnes R, Donnelly JP, Loeffler J, Jones BL, et al. (September 2019). "Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people". The Cochrane Database of Systematic Reviews. 9: CD009551. doi:10.1002/14651858.cd009551.pub4. PMC 6719256. PMID 31478559.
- ↑ Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. (January 2007). "Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia". The New England Journal of Medicine. 356 (4): 348–59. doi:10.1056/NEJMoa061094. PMID 17251531. S2CID 40335480.
- ↑ Kontoyiannis DP, Lionakis MS, Lewis RE, Chamilos G, Healy M, Perego C, et al. (April 2005). "Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases". The Journal of Infectious Diseases. 191 (8): 1350–60. doi:10.1086/428780. PMID 15776383.
- ↑ Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. (February 2008). "Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America". Clinical Infectious Diseases. 46 (3): 327–60. doi:10.1086/525258. PMID 18177225.
- ↑ Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. (August 2002). Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. "Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis". The New England Journal of Medicine. 347 (6): 408–15. doi:10.1056/NEJMoa020191. hdl:2066/185528. PMID 12167683.
- ↑ Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, et al. (May 2007). "Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial)". Clinical Infectious Diseases. 44 (10): 1289–97. doi:10.1086/514341. PMID 17443465.
- ↑ Denning DW, Park S, Lass-Florl C, Fraczek MG, Kirwan M, Gore R, et al. (May 2011). "High-frequency triazole resistance found In nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease". Clinical Infectious Diseases. 52 (9): 1123–9. doi:10.1093/cid/cir179. PMC 3106268. PMID 21467016.
- ↑ Perea S, Patterson TF (November 2002). "Antifungal resistance in pathogenic fungi". Clinical Infectious Diseases. 35 (9): 1073–80. doi:10.1086/344058. PMID 12384841.
- ↑ Bongomin F, Gago S, Oladele RO, Denning DW (October 2017). "Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision". Journal of Fungi. 3 (4): 57. doi:10.3390/jof3040057. PMC 5753159. PMID 29371573.
- ↑ Bongomin F, Batac CR, Richardson MD, Denning DW (June 2018). "A Review of Onychomycosis Due to Aspergillus Species". Mycopathologia. 183 (3): 485–493. doi:10.1007/s11046-017-0222-9. PMC 5958150. PMID 29147866.
- ↑ "Aspergillus bronchitis | Aspergillus & Aspergillosis Website". www.aspergillus.org.uk. Archived from the original on 2020-07-26. Retrieved 2021-03-01.
- ↑ Durack J, Boushey HA, Lynch SV (July 2016). "Airway Microbiota and the Implications of Dysbiosis in Asthma". Current Allergy and Asthma Reports. 16 (8): 52. doi:10.1007/s11882-016-0631-8. PMID 27393699. S2CID 29293698.
- ↑ Machado, Marina; Valerio, Maricela; Álvarez-Uría, Ana; Olmedo, María; Veintimilla, Cristina; Padilla, Belén; Villa, Sofía De la; Guinea, Jesús; Escribano, Pilar; Ruiz-Serrano, María Jesús; Reigadas, Elena; Alonso, Roberto; Guerrero, José Eugenio; Hortal, Javier; Bouza, Emilio; Muñoz, Patricia (2021). "Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity". Mycoses. 64 (2): 132–143. doi:10.1111/myc.13213. ISSN 1439-0507. Archived from the original on 2021-06-02. Retrieved 2021-05-31.
- ↑ Anderson C (2019-06-13). "World's fattest parrot, the endangered kākāpō, could be wiped out by fungal infection". The Guardian. ISSN 0261-3077. Archived from the original on 2019-06-13. Retrieved 2019-06-13.
Further reading
- Latgé JP (April 1999). "Aspergillus fumigatus and aspergillosis". Clinical Microbiology Reviews. 12 (2): 310–50. doi:10.1128/CMR.12.2.310. PMC 88920. PMID 10194462. (Review).
- Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latgé JP, Steinbach WJ (November 2014). "Aspergillus fumigatus and related species". Cold Spring Harbor Perspectives in Medicine. 5 (2): a019786. doi:10.1101/cshperspect.a019786. PMC 4315914. PMID 25377144. (Review)
External links
- USGS National Wildlife Health Center
- Aspergillus & Aspergillosis Website Archived 2021-03-15 at the Wayback Machine
- National Aspergillosis Centre, Manchester, UK Archived 2021-02-26 at the Wayback Machine
- Aspergillosis Community Website (primarily for patients and carers) Archived 2021-04-11 at the Wayback Machine
| Classification | |
|---|---|
| External resources |
.jpg.webp)
.jpg.webp)
.jpg.webp)