Mucormycosis
| Mucormycosis | |
|---|---|
| Other names: Zygomycosis[1] black fungus[2][3] | |
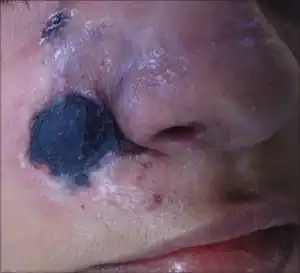 | |
| Mucormycosis: tissue destruction of sinus and skin near nose (most common type) | |
| Specialty | Infectious disease |
| Symptoms | Depends on location: runny nose, black area of skin, facial swelling, headache, fever, cough, blurred vision[4][5] |
| Complications | Blindness, thrombosis[6] |
| Usual onset | Rapid[7] |
| Duration | Around a week[7] |
| Types | Sinuses and brain, lung, stomach and intestine, skin, disseminated, miscellaneous[8] |
| Causes | Fungi of the Mucorales type[8] |
| Risk factors | Diabetes, iron overload, low white cells, cancer, organ transplant, kidney problems, immunosuppressants, long-term steroids[6] |
| Diagnostic method | Biopsy, culture, medical imaging[4] |
| Differential diagnosis | Orbital cellulitis, cavernous sinus thrombosis, aspergillosis[9] |
| Prevention | Face masks, avoiding contact with soil or water-damaged buildings, good diabetic control[6][10] |
| Treatment | Antifungal medication, surgical debridement, control sugars[6] |
| Medication | Amphotericin B, isavuconazole, posaconazole[8] |
| Prognosis | Poor[9] |
| Frequency | Rare,[8] more common in India (2020)[11] |
Mucormycosis is a serious fungal infection, generally in people with less ability to fight infection.[1] Symptoms depend on the part of the body infected.[12][13] It most commonly infects the sinuses and brain resulting in a runny nose, one sided facial swelling and pain, headache, fever, and tissue death.[4][5] Other forms of disease may infect the lungs, stomach and intestines, and skin.[5]
It is generally spread by breathing in, eating food contaminated by, or getting spores of molds of the Mucorales type in an open wound.[14] These fungi are frequently present in soil, decomposing organic matter such as rotting fruit and vegetables, and animal manure, but do not usually affect people.[15] It is not transmitted between people.[13] Risk factors include diabetes with persistently high sugars or diabetic ketoacidosis, low white cells, cancer, organ transplant, iron overload, kidney problems, long-term steroids or immunosuppressant use, and to a lesser extent in HIV/AIDS.[6][9]
Diagnosis is by biopsy and culture, with medical imaging to help determine the extent of disease.[4] It may appear similar to aspergillosis.[16] Treatment is generally with amphotericin B and surgical debridement.[8] Preventive measures include wearing a face mask in dusty areas, avoiding contact with water-damaged buildings, and protecting the skin from exposure to soil such as when gardening or certain outdoor work.[10] It tends to progress rapidly and is fatal in about half of sinus cases and almost all cases of the widespread type.[17][18]
Mucormycosis is rare, but probably underreported.[1] It affects fewer than 2 people per million people each year in San Francisco.[8] However, it is around 80 times more common in India.[19] People of any age may be affected, including premature infants.[8] The first case of mucormycosis was possibly one described by Friedrich Küchenmeister in 1855.[7] The disease has been reported in natural disasters; 2004 Indian Ocean tsunami and the 2011 Missouri tornado.[20] During the COVID-19 pandemic 2020/21, an association between mucormycosis and COVID-19 has been reported following treatment and recovery from COVID-19.[3][21] A rise in cases was particularly noted in India.[11]
Classification
Generally, mucormycosis is classified into six types according to the part of the body affected:[1][22]
- Sinuses and brain (rhinocerebral); most common in people with poorly controlled diabetes and in people who have had a kidney transplant.[13]
- Lungs (pulmonary); the most common type of mucormycosis in people with cancer and in people who have had an organ transplant or a stem cell transplant.[13]
- Stomach and intestine (gastrointestinal); more common among young premature and low birth weight infants, who have had antibiotics, surgery, or medications that lower the body's ability to fight infection.[13]
- Skin (cutaneous); after a burn, or other skin injury, in people with leukaemia, poorly controlled diabetes, Graft-versus-host disease, HIV and intravenous drug use.[13][4]
- Widespread (disseminated); when the infection spreads to other organs via the blood.[13]
- Miscellaneous or other sites involved although less commonly affected.[22]
Signs and symptoms
Signs and symptoms of Mucormycosis relate to where the infection is.[5] Infection usually begins in the mouth or nose and enters the central nervous system via the eyes.[4]
Should the fungus deposit in the nose or sinus and extend to brain, symptoms and signs may include one-sided face pain or headache.[7] There can be numbness, fever, loss of smell, a blocked nose or runny nose.[7] The person may appear to have sinusitis.[23] The face may look swollen on one side, with rapidly progressing "black lesions" across the nose or upper inside of mouth. One eye may look swollen and bulging, and vision may be blurred.[5][23][24]
Fever, cough, chest pain, and difficulty breathing, or coughing up blood, can occur when the lungs are involved.[5] A tummy ache, nausea, vomiting and bleeding can occur when the gastrointestinal tract is involved.[5][25] Affected skin may appear as a dusky reddish tender patch with a darkening centre due to tissue death.[26] There may be an ulcer and it can be very painful.[4][6][26]
Invasion into the blood vessels can result in thrombosis and subsequent death of surrounding tissue due to a loss of blood supply.[6] Widespread (disseminated) mucormycosis typically occurs in people who are already sick from other medical conditions, so it can be difficult to know which symptoms are related to mucormycosis. People with disseminated infection in the brain can develop mental status changes or coma.[27][28]
 Mucormycosis in eye
Mucormycosis in eye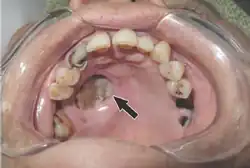 Tissue destruction of inside upper mouth
Tissue destruction of inside upper mouth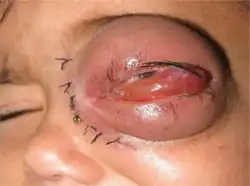 Swelling of upper and lower eyelid
Swelling of upper and lower eyelid.jpg.webp) Mucormycosis skin near eyes and nose.[29]
Mucormycosis skin near eyes and nose.[29].jpg.webp) Mucormycosis skin of forehead.[29]
Mucormycosis skin of forehead.[29]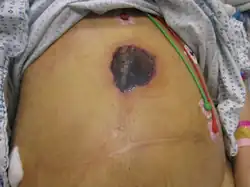 Mucormycosis skin: after liver transplantation
Mucormycosis skin: after liver transplantation
Cause
Mucormycosis is a fungal infection caused by fungi in the order Mucorales.[4] In most cases it is due to an invasion of the genera Rhizopus and Mucor, common bread molds.[30] Most fatal infections are caused by Rhizopus oryzae.[16] It is less likely due to Lichtheimia, and rarely due to Apophysomyces.[31] Others include Cunninghamella, Mortierella, and Saksenaea.[4][32]
The fungal spores are in the environment, can be found on for instance moldy bread and fruit and are breathed in frequently, but cause disease only in some people.[4] In addition to being breathed in to be deposited in the nose, sinuses and lungs, the spores can also enter the skin via blood or directly through a cut or open wound, or grow in the intestine if eaten.[13][32] Other reported causes include contaminated wound dressings.[4] Mucormycosis has been reported following the use of elastoplast and the use of tongue depressors for holding in place intravenous catheters,[4] Outbreaks have also been linked to hospital bed sheets, negative-pressure rooms, water leaks, poor ventilation, contaminated medical equipment, and building works.[33]
 Moldy bread
Moldy bread Moldy lemon
Moldy lemon Dung
Dung Mold on wet soil
Mold on wet soil Mucor
Mucor
Risk factors
Predisposing factors for mucormycosis include conditions where people are less able to fight infection, have a low neutrophil count or metabolic acidosis.[26][9] Risk factors include poorly controlled diabetes with persistently high sugars or diabetic ketoacidosis, organ transplant, iron overload, cancers such as lymphomas, kidney failure, long term corticosteroid and immunosuppressive therapy, liver disease and severe malnutrition.[10][32] Other risk factors include tuberculosis (TB),[20] deferoxamine[7] and to a lesser extent HIV/AIDS.[7][6] Cases of mucormycosis in fit and healthy people is rare.[6]
Corticosteroids are commonly used in the treatment of COVID-19 and reduce damage caused by the body's own immune system during a coronavirus infection. They are immunosuppressant and increase blood sugar levels in both diabetics and non-diabetic patients. It is thought that both these effects may contribute to cases of mucormycosis.[34][35][21]
Mechanism
Most people are frequently exposed to Mucorales without developing the disease.[32] Mucormycosis is generally spread by breathing in, eating food contaminated by, or getting spores of molds of the Mucorales type in an open wound.[14] It is not transmitted between people.[13] Once deposited, the fungus grows branch-like filaments which invade surrounding tissue and blood vessels, causing clots to form and surrounding tissues to die.[4]
The precise mechanism by which diabetics become susceptible is unclear. In vivo, a high sugar alone does not permit the growth of the fungus, but acidosis alone does.[7][6] Metabolic acidosis renders neutrophils less able to kill the fungus.[9] People with high sugars frequently have higher iron levels, also known to be a risk factor for developing mucormycosis.[6] In people on deferoxamine, the iron removed is captured by siderophores on Rhizopus species, which uses the iron to grow.[36]
Diagnosis
There is no blood test that can confirm the diagnosis.[37] Diagnosis requires early suspicion and identifying the mold in the affected tissue by biopsy and confirming it with a fungal culture.[8] Because the causative fungi occur all around, a culture alone is not decisive.[4] Tests may also include culture and direct detection of the fungus in lung fluid, blood, serum, plasma and urine.[20] Blood tests include a complete blood count to look specifically for neutropenia.[37] Other blood tests include iron levels, blood glucose, bicarbonate, and electrolytes.[37] Endoscopic examination of the nasal passages may be needed.[37] If gastrointestinal mucormycosis is suspected, a gastroscopy or colonoscopy may be needed to obtain biopsy samples.[9]
Imaging
Imaging is often performed, such as CT scan of lungs and sinuses.[38] Signs on chest CT scans, such as nodules, cavities, halo signs, pleural effusion and wedge-shaped shadows, showing invasion of blood vessels may suggest a fungal infection, but does not confirm mucormycosis.[16] A reverse halo sign in a person with a blood cancer and low neutrophil count, is highly suggestive of mucormycosis.[16] CT scan images of mucormycosis can be useful to distinguish mucormycosis of the orbit and cellulitis of the orbit, but imaging may look identical to those of aspergillosis.[16] MRI may also be useful.[39]
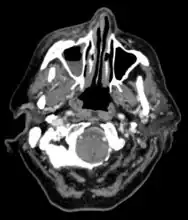 CT head (axial): invasion of right maxillary sinus (presented with double vision, swollen painful eye).
CT head (axial): invasion of right maxillary sinus (presented with double vision, swollen painful eye).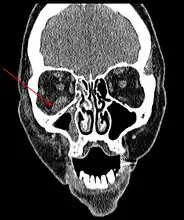 CT head (coronal) of same person.
CT head (coronal) of same person.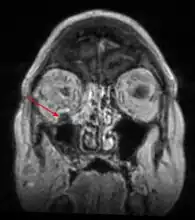 MRI head: right sided sinus involvement extending into the orbit.
MRI head: right sided sinus involvement extending into the orbit.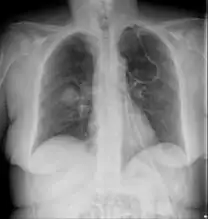 Chest X-ray: likely fungal infection left lung in an immunocompromised person
Chest X-ray: likely fungal infection left lung in an immunocompromised person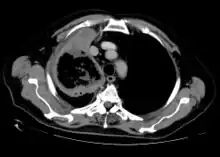 CT scan chest of person treated for acute myeloid leukaemia, presented with cough, fever and shortness of breath.
CT scan chest of person treated for acute myeloid leukaemia, presented with cough, fever and shortness of breath.
Culture and biopsy
To confirm the diagnosis, biopsy samples can be cultured.[12][37] Culture from biopsy samples does not always give a result as the organism is very fragile.[16] To precisely identify the species requires an expert.[16] The appearance of the fungus under the microscope will determine the genus and species.[37] The appearances can vary but generally show wide, ribbon-like filaments that generally don't have septa and that unlike in aspergillosis, branch at right angles, resembling "moose antlers", which may be seen to be invading blood vessels.[26]
 Sabouraud's dextrose agar showed cotton wool like colonies of Mucor
Sabouraud's dextrose agar showed cotton wool like colonies of Mucor.jpg.webp)
.jpg.webp) The angle of branching is near 90° (aspergillus branches at acute <45°).[40]
The angle of branching is near 90° (aspergillus branches at acute <45°).[40] Lactophenol cotton blue preparation showed mature sporangia of Mucor species
Lactophenol cotton blue preparation showed mature sporangia of Mucor species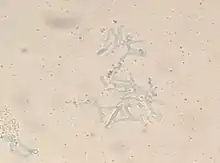 Swartz Lamkins stain of skin biopsy
Swartz Lamkins stain of skin biopsy
Other
Matrix-assisted laser desorption/ionization may be used to identify the species.[37] A blood sample from an artery may be useful to assess for metabolic acidosis.[37]
Differential diagnosis
Other filamentous fungi may however look similar.[33] It may be difficult to differentiate from aspergillosis.[41] Other possible diagnoses include anthrax, cellulitis, bowel obstruction, ecthyma gangrenosum, lung cancer, clot in lungs, sinusitis, tuberculosis and fusariosis.[42] Early in the disease, it may appear like any other sinusitis.[1]
Prevention
Preventive measures include wearing a face mask in dusty areas, washing hands, avoiding direct contact with water-damaged buildings, and protecting skin, feet and hands where there is exposure to soil or manure such as gardening or certain outdoor work.[10] In high risk groups such as organ transplant, antifungal drugs may be given as a preventative.[10]
Treatment
Treatment involves a combination of antifungal drugs, surgically removing infecting tissue and correcting underlying medical problems such as diabetic ketoacidosis.[7]
Medication
Once mucormycosis is suspected, Amphotericin B (usually the lipid formulation) at an initial dose of 1mg is initially given slowly over 10-15 minutes into a vein, then given as a once daily dose according to body weight for the next 14 days or according to response.[9][43] It may need to be continued for longer.[41] Isavuconazole and Posaconazole are alternatives.[20][44]
Surgery
Surgery can be very drastic, and in some cases of disease involving the nasal cavity and the brain, removal of infected brain tissue may be required. Removal of the palate, nasal cavity, or eye structures can be very disfiguring.[25] Sometimes more than one operation is required.[32]
Other considerations
The disease must be monitored carefully for any signs of reemergence.[32][45] Treatment also requires correcting sugar levels and improving neutrophil counts.[7] [6] Hyperbaric oxygen may be considered as an adjunctive therapy, because higher oxygen pressure increases the ability of neutrophils to kill the fungus.[6] The efficacy of this therapy is uncertain.[33]
Prognosis
It tends to progress rapidly and is fatal in about half of sinus cases, two thirds of lung cases, and almost all cases of the widespread type.[18] Skin involvement carries the lowest mortality rate of around 15%.[32] Possible complications of mucormycosis include the partial loss of neurological function, blindness and clotting of blood vessels in the brain or lung.[25]
As treatment usually requires extensive and often disfiguring facial surgery, the effect on life after surviving particularly sinus and brain involvement, is significant.[32]
Epidemiology
The true incidence and prevalence of mucormycosis may be higher than appears.[36] Mucormycosis is rare, affecting fewer than 1.7 people per million population each year in San Francisco.[8][46] It is around 80 times more prevalent in India, where it is estimated that there are around 0.14 cases per 1000 population,[19] and where its incidence has been rising.[47] Causative fungi are highly dependant on location. Apophysomyces variabilis has its highest prevalence in Asia and Lichtheimia spp. in Europe.[20] It is the third most common serious fungal infection to infect people, after aspergillosis and candidiasis.[48]
Diabetes is the main underlying disease in low and middle income countries, whereas, blood cancers and organ transplantation are the more common underlying problems in developed countries.[19] As new immunomodulating drugs and diagnostic tests are developed, the statistics for mucormycosis have been changing.[19] In addition, the figures change as new genera and species are identified, and new risk factors reported such as tuberculosis and kidney problems.[19]
History
The first case of mucormycosis was possibly one described by Friedrich Küchenmeister in 1855.[7] Fürbringer first described the disease in the lungs in 1876.[49] In 1884, Lichtheim established the development of the disease in rabbits and described two species; Mucor corymbifera and Mucor rhizopodiformis, later known as Lichtheimia and Rhizopus, respectively.[7] In 1943, its association with poorly controlled diabetes was reported in three cases with severe sinus, brain and eye involvement.[7]
In 1953, Saksenaea vasiformis, found to cause several cases, was isolated from Indian forest soil, and in 1979, P. C. Misra examined soil from an Indian mango orchard, from where they isolated Apophysomyces, later found to be a major cause of mucormycosis.[7] Several species of mucorales have since been described.[7] When cases were reported in the United States in 1955, the author thought it to be a new disease resulting from the use of antibiotics, ACTH and steroids. Until the latter half of the 20th century, the only available treatment was potassium iodide. In a review of cases involving the lungs diagnosed following flexible bronchoscopy between 1970 and 2000 , survival was found to be better in those who received combined surgery and medical treatment, mostly with amphotericin B.[49]
Naming
Arnold Paltauf coined the term 'Mycosis Mucorina' in 1885, after describing a case with systemic symptoms involving the sinus, brain and gastrointestinal tract, following which the term 'mucormycosis' became popular.[7] 'Mucormycosis' is often used interchangeably with 'zygomycosis', a term made obsolete following changes in classification of the kingdom Fungi. The former phylum Zygomycota, included Mucorales, Entomophthorales, and others. Mucormycosis describes infections caused by fungi of the order Mucorales.[41]
Society and culture
The disease has been reported in natural disasters and catastrophes; 2004 Indian Ocean tsunami and the 2011 Missouri tornado.[20][50] The first international congress on mucormycosis was held in Chicago in 2010, set up by the Hank Schueuler 41 & 9 Foundation, which was established in 2008 for the research of children with leukaemia and fungal infections.[7] A cluster of infections occurred in the wake of the 2011 Joplin tornado. By July 19, 2011 a total of 18 suspected cases of mucormycosis of the skin had been identified, of which 13 were confirmed. A confirmed case was defined as 1) necrotizing soft-tissue infection requiring antifungal treatment or surgical debridement in a person injured in the tornado, 2) with illness onset on or after May 22 and 3) positive fungal culture or histopathology and genetic sequencing consistent with a mucormycete. No additional cases related to that outbreak were reported after June 17. Ten people required admission to an intensive-care unit, and five died.[51][52]
In 2014, details of a lethal mucormycosis outbreak which occurred in 2008 emerged after television and newspaper reports responded to an article in a pediatric medical journal.[53][54] Contaminated hospital linen was found to be spreading the infection. A 2018 study found many freshly laundered hospital linens delivered to U.S. transplant hospitals were contaminated with Mucorales.[55]
COVID-19–association
During the COVID-19 pandemic, the disease became popularly known as "black fungus", following reported cases in India.[2][34] A number of cases of aspergillosis and candidiasis linked to immunosuppressive treatment for COVID-19 were also reported during the COVID-19 pandemic in India in 2020.[3][39] One review in early 2021 relating to the association of mucormycosis and COVID-19 reported eight cases of mucormycosis; three from the US, two from India, and one case each from Brazil, Italy, and the UK.[21] The commonest underlying medical condition was diabetes.[21] Most had been in hospital with severe breathing problems due to COVID-19, had recovered, and developed mucormycosis 10-14 days following treatment for COVID-19. Five had abnormal kidney function tests, three involved the sinus, eye and brain, three the lungs, one the gastrointestinal tract, and in one the disease was widespread.[21] In two of the seven deaths, the diagnosed of mucormycosis was made at postmortem.[21] That three had no traditional risk factors led the authors to question the use of steroids and Immunosuppressive drugs.[21] A review of 43 cases of COVID-19–associated mucormycosis in April 2021 showed that where Mucorales were identified, the Rhizopus species was isolated in more than three-quarters.[56] In a review of COVID-19 related eye problems, mucormycosis affecting the eyes was reported to occur up to several weeks following recovery from COVID-19.[39] Another review that month, of global cases of mucormycosis associated with COVID-19 reported 101 cases; 82 cases from India and 19 from the rest of the world. Males, people with pre-existing diabetes and those who had taken corticosteroids were affected more frequently. The authors concluded that the prevalence of diabetes and widespread use of corticosteroid on a background of COVID-19 appeared to increase mucormycosis, and appealed for good sugar controls and stricter use of steroids in people with COVID-19.[11]
Other animals
Mucormycosis in other animals is similar, in terms of frequency and types, as in people.[57] Cases have been described in cats, dogs, cows, horses, dolphin, bison, and seals.[57]
References
- 1 2 3 4 5 Kontoyiannis, Dimitrios P. (2020). "320. Mucormycosis". In Goldman, Lee; Schafer, Andrew I. (eds.). Goldman-Cecil Medicine. Vol. 2 (26th ed.). Philadelphia: Elsevier. p. 2056-2058. ISBN 978-0-323-55087-1. Archived from the original on May 2, 2023. Retrieved May 26, 2022.
- 1 2 Dyer, Owen (May 13, 2021). "Covid-19: India sees record deaths as "black fungus" spreads fear". BMJ (Clinical research ed.). 373: n1238. doi:10.1136/bmj.n1238. ISSN 1756-1833. PMID 33985993. Archived from the original on May 26, 2021. Retrieved May 29, 2021.
- 1 2 3 Quarterly Current Affairs Vol. 4 - October to December 2020 for Competitive Exams. Vol. 4. Disha Publications. 2020. p. 173. ISBN 978-93-90486-29-8. Archived from the original on May 25, 2021. Retrieved May 16, 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Grossman, Marc E.; Fox, Lindy P.; Kovarik, Carrie; Rosenbach, Misha (2012). "1. Subcutaneous and deep mycoses: Zygomucosis/Mucormycosis". Cutaneous Manifestations of Infection in the Immunocompromised Host (2nd ed.). Springer. pp. 51–58. ISBN 978-1-4419-1577-1. Archived from the original on May 25, 2021. Retrieved May 16, 2021.
- 1 2 3 4 5 6 7 "Symptoms of Mucormycosis". www.cdc.gov. January 14, 2021. Archived from the original on May 14, 2021. Retrieved May 25, 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Spellberg B, Edwards J, Ibrahim A (2005). "Novel perspectives on mucormycosis: pathophysiology, presentation, and management". Clin. Microbiol. Rev. 18 (3): 556–69. doi:10.1128/CMR.18.3.556-569.2005. PMC 1195964. PMID 16020690.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Chander, Jagdish (2018). "26. Mucormycosis". Textbook of Medical Mycology (4th ed.). New Delhi: Jaypee Brothers Medical Publishers Ltd. pp. 534–596. ISBN 978-93-86261-83-0. Archived from the original on May 25, 2021. Retrieved May 22, 2021.
- 1 2 3 4 5 6 7 8 9 "Mucormycosis". NORD (National Organization for Rare Disorders). Archived from the original on May 26, 2021. Retrieved May 25, 2021.
- 1 2 3 4 5 6 7 Hernández, Jorge L.; Buckley, Clifford J. (July 25, 2021). "Mucormycosis". StatPearls. StatPearls Publishing. PMID 31335084. Archived from the original on January 13, 2022. Retrieved May 4, 2022.
- 1 2 3 4 5 "People at Risk For Mucormycosis and prevention". www.cdc.gov. February 2, 2021. Archived from the original on May 14, 2021. Retrieved May 25, 2021.
- 1 2 3 Singh, Awadhesh Kumar; Singh, Ritu; Joshi, Shashank R.; Misra, Anoop (May 21, 2021). "Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India". Diabetes & Metabolic Syndrome. doi:10.1016/j.dsx.2021.05.019. ISSN 1871-4021. Archived from the original on June 10, 2021. Retrieved June 9, 2021.
- 1 2 "ICD-11 - ICD-11 for Mortality and Morbidity Statistics". icd.who.int. Archived from the original on August 1, 2018. Retrieved May 25, 2021.
- 1 2 3 4 5 6 7 8 9 "About Mucormycosis". www.cdc.gov. May 25, 2021. Archived from the original on May 14, 2021. Retrieved May 12, 2021.
- 1 2 Reid, Gail; Lynch, Joseph P.; Fishbein, Michael C.; Clark, Nina M. (February 2020). "Mucormycosis". Seminars in Respiratory and Critical Care Medicine. 41 (1): 99–114. doi:10.1055/s-0039-3401992. ISSN 1098-9048. PMID 32000287. Archived from the original on May 22, 2021. Retrieved May 18, 2021.
- ↑ "Where Mucormycosis Comes From". www.cdc.gov. February 1, 2021. Archived from the original on May 15, 2021. Retrieved May 25, 2021.
- 1 2 3 4 5 6 7 Thornton, Christopher R. (2020). "1. Detection of the 'Big Five' mold killers of humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes". In Gadd, Geoffrey M.; Sariaslani, Sima (eds.). Advances in Applied Microbiology. Academic Press. pp. 4–22. ISBN 978-0-12-820703-1. Archived from the original on May 25, 2021. Retrieved May 25, 2021.
- ↑ "Orphanet: Zygomycosis". www.orpha.net. Archived from the original on May 13, 2021. Retrieved May 13, 2021.
- 1 2 "Mucormycosis Statistics | Mucormycosis | Fungal Diseases | CDC". www.cdc.gov. May 5, 2020. Archived from the original on May 21, 2021. Retrieved May 25, 2021.
- 1 2 3 4 5 Skiada, Anna; Pavleas, Ioannis; Drogari-Apiranthitou, Maria (November 2, 2020). "Epidemiology and Diagnosis of Mucormycosis: An Update". Journal of Fungi. 6 (4). doi:10.3390/jof6040265. ISSN 2309-608X. PMC 7711598. PMID 33147877.
- 1 2 3 4 5 6 Dannaoui, Eric; Lackner, Michaela (March 2020). "Special Issue: Mucorales and Mucormycosis". Journal of Fungi. 6 (1): 6. doi:10.3390/jof6010006. PMC 7151165. Archived from the original on May 22, 2021. Retrieved May 18, 2021.
- 1 2 3 4 5 6 7 Garg, Deepak; Muthu, Valliappan; Sehgal, Inderpaul Singh; Ramachandran, Raja; Kaur, Harsimran; Bhalla, Ashish; Puri, Goverdhan D.; Chakrabarti, Arunaloke; Agarwal, Ritesh (May 1, 2021). "Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature". Mycopathologia. 186 (2): 289–298. doi:10.1007/s11046-021-00528-2 (inactive May 25, 2021). ISSN 1573-0832. PMC 7862973. PMID 33544266.
{{cite journal}}: CS1 maint: DOI inactive as of May 2021 (link) - 1 2 Riley, Treavor T.; Muzny, Christina A.; Swiatlo, Edwin; Legendre, Davey P. (September 2016). "Breaking the Mold: A Review of Mucormycosis and Current Pharmacological Treatment Options". The Annals of Pharmacotherapy. 50 (9): 747–757. doi:10.1177/1060028016655425. ISSN 1542-6270. PMID 27307416. Archived from the original on April 12, 2021. Retrieved May 29, 2021.
- 1 2 McDonald, Philip J. "Mucormycosis (Zygomycosis) Clinical Presentation: History and Physical Examination". emedicine.medscape.com. Archived from the original on May 25, 2021. Retrieved May 28, 2021.
- ↑ Lee, Scott (2001). BrainChip for Microbiology. Blackwell Science. p. 70. ISBN 0-632-04568-X. Archived from the original on May 25, 2021. Retrieved May 22, 2021.
- 1 2 3 "MedlinePlus Medical Encyclopedia: Mucormycosis". Archived from the original on May 20, 2008. Retrieved May 19, 2008.
- 1 2 3 4 5 Johnstone, Ronald B. (2017). "25. Mycoses and Algal infections". Weedon's Skin Pathology Essentials (2nd ed.). Elsevier. p. 461. ISBN 978-0-7020-6830-0. Archived from the original on May 25, 2021. Retrieved May 25, 2021.
- ↑ Lewis, Russell E; Kontoyiannis, Dimitrios P (September 2013). "Epidemiology and treatment of mucormycosis". Future Microbiology. 8 (9): 1163–1175. doi:10.2217/fmb.13.78. ISSN 1746-0913. Archived from the original on August 28, 2021. Retrieved May 12, 2021.
- ↑ Spellberg, Brad; Edwards, John; Ibrahim, Ashraf (2005). "Novel Perspectives on Mucormycosis: Pathophysiology, Presentation, and Management". Clinical Microbiology Reviews. 18 (3): 556–569. doi:10.1128/cmr.18.3.556-569.2005. ISSN 0893-8512. PMC 1195964. Archived from the original on August 28, 2021. Retrieved May 12, 2021.
- 1 2 "Zygomycosis | DermNet NZ". dermnetnz.org. Archived from the original on April 23, 2021. Retrieved May 16, 2021.
- ↑ Lee, Soo Chan; Idmurm, Alexander (2018). "8. Fingal sex: The Mucoromycota". In Heitman, Joseph; Howlett, Barbara J.; Crous, Pedro W.; Stukenbrock, Eva H.; James, Timothy Yong; Gow, Neil A. R. (eds.). The Fungal Kingdom. Wiley. pp. 177–192. ISBN 978-1-55581-958-3. Archived from the original on May 25, 2021. Retrieved May 24, 2021.
- ↑ Martínez-Herrera, Erick; Frías-De-León, María Guadalupe; Julián-Castrejón, Angélica; Cruz-Benítez, Luis; Xicohtencatl-Cortes, Juan; Hernández-Castro, Rigoberto (August 18, 2020). "Rhino-orbital mucormycosis due to Apophysomyces ossiformis in a patient with diabetes mellitus: a case report". BMC Infectious Diseases. 20 (1): 614. doi:10.1186/s12879-020-05337-4. ISSN 1471-2334. PMC 7437167. Archived from the original on May 25, 2021. Retrieved May 24, 2021.
- 1 2 3 4 5 6 7 8 McDonald, Philip J. (September 10, 2018). "Mucormycosis (Zygomycosis): Background, Etiology and Pathophysiology, Epidemiology". Medscape. Archived from the original on December 2, 2008. Retrieved May 24, 2021.
- 1 2 3 "For Healthcare Professionals | Mucormycosis | CDC". www.cdc.gov. June 17, 2020. Archived from the original on May 21, 2021. Retrieved May 25, 2021.
- 1 2 Biswas, Soutik (May 9, 2021). "Mucormycosis: The 'black fungus' maiming Covid patients in India". BBC News. British Broadcasting Corporation. Archived from the original on May 21, 2021. Retrieved May 11, 2021.
- ↑ Koehler, Philipp; Bassetti, Matteo; Chakrabarti, Arunaloke; Chen, Sharon C A; Colombo, Arnaldo Lopes; Hoenigl, Martin; Klimko, Nikolay; Lass-Flörl, Cornelia; Oladele, Rita O; Vinh, Donald C; Zhu, Li-Ping (December 2020). "Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance". The Lancet Infectious Diseases. doi:10.1016/s1473-3099(20)30847-1. ISSN 1473-3099. PMC 7833078. PMID 33333012.
- 1 2 Prakash, Hariprasath; Chakrabarti, Arunaloke (March 2019). "Global Epidemiology of Mucormycosis". Journal of Fungi. 5 (1): 26. doi:10.3390/jof5010026. PMID 30901907. Archived from the original on June 2, 2021. Retrieved June 2, 2021.
- 1 2 3 4 5 6 7 8 McDonald, Philip J. "Mucormycosis (Zygomycosis) Workup: Approach Considerations, Laboratory Tests, Radiologic Studies". emedicine.medscape.com. Archived from the original on May 25, 2021. Retrieved May 25, 2021.
- ↑ "Diagnosis and Testing of Mucormycosis | Mucormycosis | CDC". www.cdc.gov. January 14, 2021. Archived from the original on May 21, 2021. Retrieved May 22, 2021.
- 1 2 3 Sen M, Honavar SG, Sharma N, Sachdev MS (2021). "COVID-19 and Eye: A review of ophthalmic manifestations of COVID-19". Indian Journal of Ophthalmology. 69 (3): 488–509. doi:10.4103/ijo.IJO_297_21. PMC 7942063. PMID 33595463.
- 1 2 "Mucormycosis pathology | DermNet NZ". dermnetnz.org. Archived from the original on January 19, 2021. Retrieved May 23, 2021.
- 1 2 3 Cornely, Oliver A. (December 1, 2019). "Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium". The Lancet Infectious Diseases. 19 (12): e405–e421. doi:10.1016/S1473-3099(19)30312-3. ISSN 1473-3099. PMID 31699664. Archived from the original on August 28, 2021. Retrieved May 22, 2021. (several authors)
- ↑ McDonald, Philip J . "Mucormycosis (Zygomycosis) Differential Diagnoses". emedicine.medscape.com. Archived from the original on May 25, 2021. Retrieved May 25, 2021.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 629-635. ISBN 978-0-85711-369-6.
- ↑ McDonald, Philip J. (September 10, 2018). "What is the role of isavuconazole (Cresemba) in the treatment of mucormycosis (zygomycosis)?". www.medscape.com. Archived from the original on May 21, 2021. Retrieved May 25, 2021.
- ↑ Rebecca J. Frey. "Mucormycosis". Health A to Z. Archived from the original on May 18, 2008. Retrieved May 19, 2008.
- ↑ "Mucormycosis Statistics | Mucormycosis | Fungal Diseases | CDC". www.cdc.gov. June 5, 2020. Archived from the original on May 22, 2021. Retrieved May 22, 2021.
- ↑ Vallabhaneni, Snigdha; Mody, Rajal K.; Walker, Tiffany; Chiller, Tom (2016). "1. The global burden of fungal disease". In Sobel, Jack; Ostrosky-Zeichner, Luis (eds.). Fungal Infections, An Issue of Infectious Disease Clinics of North America. Philadelphia: Elsevier. pp. 5–12. ISBN 978-0-323-41649-8. Archived from the original on June 2, 2021. Retrieved May 29, 2021.
- ↑ Petrikkos, George; Skiada, Anna; Lortholary, Olivier; Roilides, Emmanuel; Walsh, Thomas J.; Kontoyiannis, Dimitrios P. (February 1, 2012). "Epidemiology and Clinical Manifestations of Mucormycosis". Clinical Infectious Diseases. 54 (suppl_1): S23–S34. doi:10.1093/cid/cir866. ISSN 1537-6591. Archived from the original on August 28, 2021. Retrieved May 12, 2021.
- 1 2 Yamin, Hasan S.; Alastal, Amro Y.; Bakri, Izzedin (January 2017). "Pulmonary Mucormycosis Over 130 Years: A Case Report and Literature Review". Turkish Thoracic Journal. 18 (1): 1–5. doi:10.5152/TurkThoracJ.2017.16033. ISSN 2148-7197. PMC 5783164. PMID 29404149.
- ↑ Fanfair, Robyn Neblett; et al. (July 29, 2011). "Notes from the Field: Fatal Fungal Soft-Tissue Infections After a Tornado – Joplin, Missouri, 2011". MMWR Weekly. 60 (29): 992. Archived from the original on January 13, 2017. Retrieved September 10, 2017.
- ↑ Williams, Timothy (June 10, 2011) Rare Infection Strikes Victims of a Tornado in Missouri Archived September 2, 2017, at the Wayback Machine. New York Times.
- ↑ Neblett Fanfair, Robyn; Benedict, Kaitlin; Bos, John; Bennett, Sarah D.; Lo, Yi-Chun; Adebanjo, Tolu; Etienne, Kizee; Deak, Eszter; Derado, Gordana; Shieh, Wun-Ju; Drew, Clifton; Zaki, Sherif; Sugerman, David; Gade, Lalitha; Thompson, Elizabeth H.; Sutton, Deanna A.; Engelthaler, David M.; Schupp, James M.; Brandt, Mary E.; Harris, Julie R.; Lockhart, Shawn R.; Turabelidze, George; Park, Benjamin J. (December 6, 2012). "Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011". The New England Journal of Medicine. 367 (23): 2214–2225. doi:10.1056/NEJMoa1204781. ISSN 1533-4406. PMID 23215557. Archived from the original on May 24, 2021. Retrieved May 22, 2021.
- ↑ Catalanello, Rebecca (April 16, 2014). "Mother believes her newborn was the first to die from fungus at Children's Hospital in 2008". NOLA.com. Archived from the original on April 1, 2018. Retrieved April 17, 2014.
- ↑ "5 Children's Hospital patients died in 2008, 2009 after contact with deadly fungus". Archived from the original on January 6, 2019. Retrieved April 18, 2014.
We acknowledge that Children's Hospital is Hospital A in an upcoming article in The Pediatric Infectious Disease Journal. The safety and well-being of our patients are our top priorities, so as soon as a problem was suspected, the State Health Department and CDC were notified and invited to assist in the investigation. The hospital was extremely aggressive in trying to isolate and then eliminate the source of the fungus.
- ↑ Sundermann, Alexander; et al. (2018). "How Clean Is the Linen at My Hospital? The Mucorales on Unclean Linen Discovery Study of Large United States Transplant and Cancer Centers". Clinical Infectious Diseases. 68 (5): 850–853. doi:10.1093/cid/ciy669. PMC 6765054. PMID 30299481.
- ↑ John, Teny M.; Jacob, Ceena N.; Kontoyiannis, Dimitrios P. (April 15, 2021). "When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis". Journal of Fungi. 7 (4). doi:10.3390/jof7040298. ISSN 2309-608X. PMID 33920755. Archived from the original on May 15, 2021. Retrieved June 5, 2021.
- 1 2 Seyedmousavi, Seyedmojtaba; Bosco, Sandra de M G; de Hoog, Sybren; Ebel, Frank; Elad, Daniel; Gomes, Renata R; Jacobsen, Ilse D; Jensen, Henrik E; Martel, An; Mignon, Bernard; Pasmans, Frank; Piecková, Elena; Rodrigues, Anderson Messias; Singh, Karuna; Vicente, Vania A; Wibbelt, Gudrun; Wiederhold, Nathan P; Guillot, Jacques (April 1, 2018). "Fungal infections in animals: a patchwork of different situations". Medical Mycology. 56 (suppl_1): S165–S187. doi:10.1093/mmy/myx104. ISSN 1369-3786. Archived from the original on May 25, 2021. Retrieved May 22, 2021.
Further reading
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. (2019). "Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium" (PDF). The Lancet. Infectious Diseases. 19 (12): e405–e421. doi:10.1016/S1473-3099(19)30312-3. PMID 31699664. Archived (PDF) from the original on March 2, 2021. Retrieved June 2, 2021.
External links
| Classification | |
|---|---|
| External resources |
|