Farglitazar
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
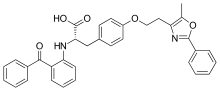
| Formula | C34H30N2O5 |
| Molar mass | 546.623 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Farglitazar is a peroxisome proliferator-activated receptor agonist which was formerly under development by GlaxoSmithKline, but has never been marketed. It progressed to phase II clinical trials for the treatment of hepatic fibrosis, but failed to show efficacy.[1] After reaching phase III for type 2 diabetes, further development was discontinued.[2][3]
References
- ↑ McHutchison J, Goodman Z, Patel K, Makhlouf H, Rodriguez-Torres M, Shiffman M, et al. (April 2010). "Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection". Gastroenterology. 138 (4): 1365–73, 1373.e1-2. doi:10.1053/j.gastro.2009.12.003. PMID 20004661.
- ↑ "Farglitazar". Adis Insight. Springer Nature Switzerland AG.
- ↑ "Farglitazar". pharmacodia.com.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.