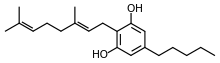
Cannabigerol
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.485 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Cannabigerol (CBG) is one of more than 120 identified cannabinoid compounds found in the plant genus Cannabis.[1][2] Cannabigerol is the decarboxylated form of cannabigerolic acid, the parent molecule from which other cannabinoids are synthesized. Cannabigerol is a minor constituent of cannabis.[3] During plant growth, most of the cannabigerol is converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD), leaving about 1% cannabigerol in the plant.[4]
Biosynthesis

The biosynthesis of cannabigerol begins by loading hexanoyl-CoA onto a polyketide synthase assembly protein and subsequent condensation with three molecules of malonyl-CoA.[5] This polyketide is cyclized to olivetolic acid via olivetolic acid cyclase, and then prenylated with a ten carbon isoprenoid precursor, geranyl pyrophosphate, using an aromatic prenyltransferase enzyme, geranyl-pyrophosphate—olivetolic acid geranyltransferase, to biosynthesize cannabigerolic acid, which can then be decarboxylated to yield cannabigerol.[3][6]
Research
As of 2019, no clinical research has been conducted to test the specific effects of cannabigerol in humans.[7]
Cannabigerol is under laboratory research to determine its pharmacological properties and potential effects in disease conditions.[7][8] Contrary to the major psychoactive cannabinoid THC, cannabigerol antagonizes CB1 receptors and is both an α2-adrenergic receptor agonist and moderate 5-HT1A receptor antagonist.[7][9] Cannabigerol displays CB1 and CB2 binding affinity,[7][8][10] and has been evaluated in laboratory models of colitis.[11]
Legal status
Cannabigerol is not scheduled by the UN Convention on Psychotropic Substances. In the United States, CBG derived from cannabis is illegal under the Controlled Substances Act, while CBG derived from hemp is legal. [12]
See also
References
- ↑ ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A (2017). Phytochemistry of Cannabis sativa L. Progress in the Chemistry of Organic Natural Products. Vol. 103. pp. 1–36. doi:10.1007/978-3-319-45541-9_1. ISBN 978-3-319-45539-6. PMID 28120229.
- ↑ Turner SE, Williams CM, Iversen L, Whalley BJ (2017). "Molecular Pharmacology of Phytocannabinoids". Progress in the Chemistry of Organic Natural Products. 103: 61–101. doi:10.1007/978-3-319-45541-9_3. ISBN 978-3-319-45539-6. PMID 28120231.
- 1 2 Morales P, Reggio PH, Jagerovic N (2017). "An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol". Frontiers in Pharmacology. 8: 422. doi:10.3389/fphar.2017.00422. PMC 5487438. PMID 28701957.
- ↑ Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–31. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ↑ Gagne SJ, Stout JM, Liu E, Boubakir Z, Clark SM, Page JE (July 2012). "Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides". Proceedings of the National Academy of Sciences of the United States of America. 109 (31): 12811–6. Bibcode:2012PNAS..10912811G. doi:10.1073/pnas.1200330109. PMC 3411943. PMID 22802619.
- ↑ Fellermeier M, Zenk MH (May 1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters. 427 (2): 283–5. doi:10.1016/s0014-5793(98)00450-5. PMID 9607329. S2CID 7777284.
- 1 2 3 4 "Cannabigerol; ID 5315659". PubChem, National Library of Medicine, US National Institutes of Health. 11 May 2019. Retrieved 12 May 2019.
- 1 2 Morales P, Hurst DP, Reggio PH (2017). "Molecular Targets of the Phytocannabinoids: A Complex Picture". Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. Vol. 103. pp. 103–131. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6. PMC 5345356. PMID 28120232.
- ↑ Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG (January 2010). "Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist". British Journal of Pharmacology. 159 (1): 129–41. doi:10.1111/j.1476-5381.2009.00515.x. PMC 2823359. PMID 20002104.
- ↑ Zagzoog A, et al. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci Rep 2020; 10: 20405. doi:10.1038/s41598-020-77175-y
- ↑ Couch DG, Maudslay H, Doleman B, Lund JN, O'Sullivan SE (March 2018). "The Use of Cannabinoids in Colitis: A Systematic Review and Meta-Analysis" (PDF). Inflammatory Bowel Diseases. 24 (4): 680–697. doi:10.1093/ibd/izy014. PMID 29562280.
- ↑ "USC > Title 21 > Chapter 13 > Subchapter I > Part A > § 802. Definitions: (16)" (PDF). Government Publishing Office - US Code. 2016.
| Phytocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthetic cannabinoid receptor agonists / neocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric CBR ligands |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Endocannabinoid enhancers (inactivation inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Anticannabinoids (antagonists/inverse agonists/antibodies) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||
| Enzyme (modulators) |
| ||||||||||||
| Others |
| ||||||||||||
| |||||||||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
TRP channel modulators | |||||
|---|---|---|---|---|---|
| TRPA |
| ||||
| TRPC |
| ||||
| TRPM |
| ||||
| TRPML |
| ||||
| TRPP |
| ||||
| TRPV |
| ||||
See also: Receptor/signaling modulators • Ion channel modulators | |||||