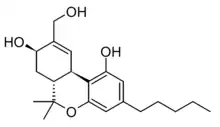
8,11-Dihydroxytetrahydrocannabinol
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H30O4 |
| Molar mass | 346.467 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
8,11-Dihydroxytetrahydrocannabinol (8β,11-diOH-Δ9-THC) is an active metabolite of THC, the main active component of cannabis. The 8β enantiomer retains psychoactive effects in animal studies with only slightly lower potency than THC, while the 8α enantiomer is much weaker. Both enantiomers have a shorter half-life in the body than 11-Hydroxy-THC, making 8,11-dihydroxy-THC potentially useful for drug testing to distinguish between recent cannabis use and use longer in the past.[1][2][3]
See also
References
- ↑ Pitt CG, Fowler MS, Sathe S, Srivastava SC, Williams DL (June 1975). "Synthesis of metabolites of delta-9-tetrahydrocannabinol". Journal of the American Chemical Society. 97 (13): 3798–3802. doi:10.1021/ja00846a040. PMID 1141589.
- ↑ Järbe TU, Mathis DA (1991). "Discriminative stimulus functions of cannabinoids/cannabimimetics". Drug Discrimination: Applications to Drug Abuse Research. NIDA Research Monograph. pp. 75–99. PMID 1369683.
- ↑ Gasse A, Pfeiffer H, Köhler H, Schürenkamp J (January 2018). "8β-OH-THC and 8β,11-diOH-THC-minor metabolites with major informative value?". International Journal of Legal Medicine. 132 (1): 157–164. doi:10.1007/s00414-017-1692-5. PMID 28983686.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.