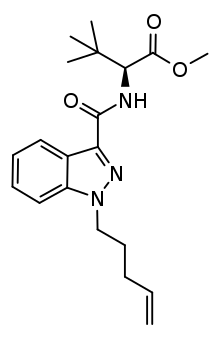
MDMB-4en-PINACA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H27N3O3 |
| Molar mass | 357.454 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
MDMB-4en-PINACA (also incorrectly known as 5-CL-ADB-A) is an indazole-based synthetic cannabinoid that has been sold online as a designer drug.[1][2][3] MDMB-4en-PINACA differs from 5F-MDMB-PINACA due to replacement of 5-fluoropentyl with a pent-4-ene moiety (4-en).[4]
It acts as a potent agonist of the CB1 receptor with an EC50 value of 2.47 nM.[5][6][7][8]
Legal status
Sweden's public health agency suggested classifying MDMB-4en-PINACA as a hazardous substance, on December 18, 2019.[9]
See also
- ADB-PINACA
- ADB-4en-PINACA
- MDMB-CHMICA
- MDMB-CHMINACA
- MDMB-FUBINACA
- MMB-4en-PICA
References
- ↑ Watanabe S, Vikingsson S, Åstrand A, Gréen H, Kronstrand R (December 2019). "Biotransformation of the New Synthetic Cannabinoid with an Alkene, MDMB-4en-PINACA, by Human Hepatocytes, Human Liver Microsomes, and Human Urine and Blood". The AAPS Journal. 22 (1): 13. doi:10.1208/s12248-019-0381-3. PMID 31848852. S2CID 209393242.
- ↑ "MDMB-4en-PINACA". www.caymanchem.com.
- ↑ Erol Ozturk Y, Yeter O (January 2021). "In Vitro Phase I Metabolism of the Recently Emerged Synthetic MDMB-4en-PINACA and Its Detection in Human Urine Samples". Journal of Analytical Toxicology. 44 (9): 976–984. doi:10.1093/jat/bkaa017. PMID 32091101.
- ↑ "ANALYTICAL REPORT MDMB-PINACA N1-pentyl-4-en isomer" (PDF). www.policija.si.
- ↑ Krotulski AJ, Cannaert A, Stove C, Logan BK (February 2021). "The next generation of synthetic cannabinoids: Detection, activity, and potential toxicity of pent-4en and but-3en analogues including MDMB-4en-PINACA". Drug Testing and Analysis. 13 (2): 427–438. doi:10.1002/dta.2935. PMID 32997377.
- ↑ Cannaert A, Sparkes E, Pike E, Luo JL, Fang A, Kevin RC, et al. (December 2020). "Synthesis and in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA". ACS Chemical Neuroscience. 11 (24): 4434–4446. doi:10.1021/acschemneuro.0c00644. PMID 33253529.
- ↑ Pike E, Grafinger KE, Cannaert A, Ametovski A, Luo JL, Sparkes E, et al. (July 2021). "Systematic evaluation of a panel of 30 synthetic cannabinoid receptor agonists structurally related to MMB-4en-PICA, MDMB-4en-PINACA, ADB-4en-PINACA, and MMB-4CN-BUTINACA using a combination of binding and different CB1 receptor activation assays: Part I-Synthesis, analytical characterization, and binding affinity for human CB1 receptors". Drug Testing and Analysis. 13 (7): 1383–1401. doi:10.1002/dta.3037. PMID 33787091.
- ↑ Grafinger KE, Cannaert A, Ametovski A, Sparkes E, Cairns E, Banister SD, et al. (July 2021). "Systematic evaluation of a panel of 30 synthetic cannabinoid receptor agonists structurally related to MMB-4en-PICA, MDMB-4en-PINACA, ADB-4en-PINACA, and MMB-4CN-BUTINACA using a combination of binding and different CB1 receptor activation assays-Part II: Structure activity relationship assessment via a β-arrestin recruitment assay". Drug Testing and Analysis. 13 (7): 1402–1411. doi:10.1002/dta.3035. PMID 33769699.
- ↑ "Tjugotre ämnen föreslås klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 18 December 2019.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.